浙贝母中分离纯化贝母碱和去氢贝母碱检测方案(高速逆流色谱)
检测样品 中药材和饮片
检测项目 含量测定
方案详情文
智能文字提取功能测试中
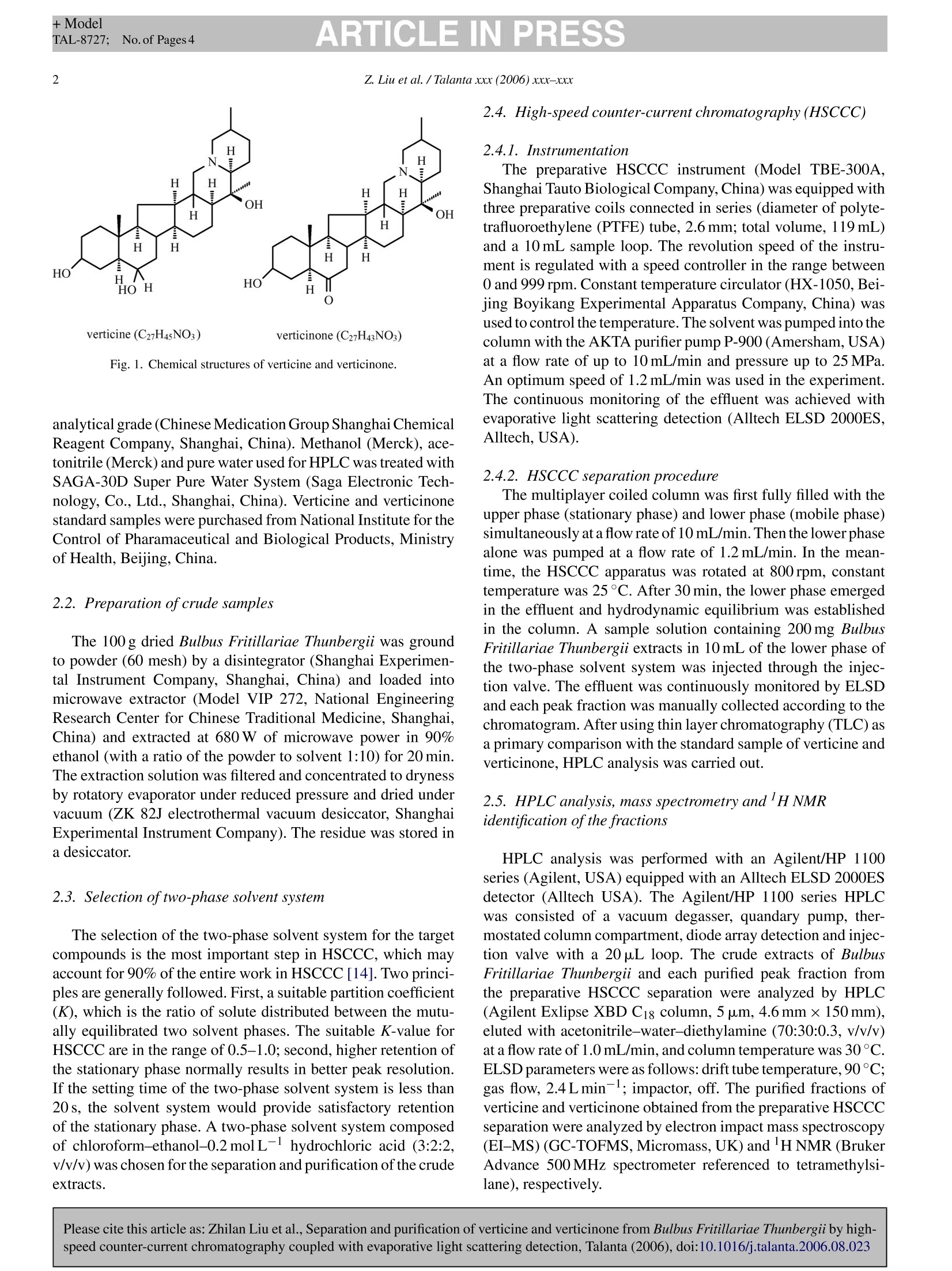
+ModelTAL-8727:No. of Pages 4ARTICLE IN PRESSAvailable online at www.sciencedirect.comScienceDirect +ModelTAL-8727:No. of Pages 4ARTICLE IN PRESSZ. Liu et al. /Talanta xxx (2006) xxx-xxx Please cite this article as: Zhilan Liu et al., Separation and purification of verticine and verticinone from Bulbus Fritillariae Thunbergii by high-speed counter-current chromatography coupled with evaporative light scattering detection, Talanta (2006), doi:10.1016/j.talanta.2006.08.023 Talanta ELSEVIER Talanta xxx (200 6) xxx-xxx www.elsevier.com/locate/talanta Separation and purification of verticine and verticinone from BulbusFritillariae Thunbergii by high-speed counter-current chromatographycoupled with evaporative light scattering detection Zhilan Liu, Yan Jin, Pingniang Shen,Juan Wang, Yongjia Shen a* a Laboratory for Advanced Materials, Institute of Fine Chemicals, East China University of Science and Technology, Shanghai 200237, China National Engineering Research Center for Traditional Chinese Medicine, Shanghai 201203, China Received 7 July 2006; received in revised form 19 August 2006; accepted 20 August 2006 Abstract High-speed counter-current chromatography (HSCCC) coupled with evaporative light scattering detection (ELSD) was successfully applied topreparative separation and purification of verticine and verticinone from crude extracts of Bulbus Fritillariae Thunbergii by a one-step separation,using chloroform-ethanol-0.2 mol L- hydrochloric acid (3:2:2, v/v/v) as a solvent system. HPLC analysis of the fractions collected on thepreparative HSCCC of 200 mg of crude extracts showed that the purity of verticine (25.6mg) was 96.8% and that of verticinone (10.3 mg) was95.4%. The chemical identities of these components were confirmed by 'HNMR and EI-MS. O 2006 Elsevier B.V. All rights reserved. Keywords: Counter-current chromatography; Bulbus Fritillariae Thunbergii; Verticine; Verticinone; Evaporative light scattering detection 1. Introduction Bulbus Fritillariae Thunbergii (Chinese name zhebeimu) isa famous Chinese medicine, which is derived from the bulbs ofthe genus Fritillaria thunbergii Miq. (Liliaceae) [1]. It has beenused as one of the most important antitussive and expectorantdrugs for more than 2000 years [2]. Extensive chemical studieshave been conducted by many research groups [3-6]. A num-ber of ingredients were found in Bulbus Fritillariae Thunbergiiincluding isosteroidal alkaloids, steroidal alkaloids and non-alkaloids. Furthermore, pharmacological studies demonstratethat isosteroidal alkaloids are the primary active ingredientsresponsible for the antitussive activity. Among these alkaloids,verticine and verticinone (Fig. 1) are the most representative[7]. The conventional method of purifying verticine and verti-cinone was to utilize column chromatography, which requiredseveral steps, and resulted in low recovery yields [8]. High- ( * Corresponding a u thor. T e l.: +86 21 64252967; f a x: +86 2 1 64252967.E-mail address: yjshe n @ecust.edu.cn ( Y. Shen). ) speed counter-current chromatography (HSCCC), being as asupport free liquid-liquid partition chromatography, eliminatesirreversible adsorption of sample onto the solid support. Thismethod has been successfully applied to the separation andpurification of various natural products [9-12], and severalreports have tried the HSCCC coupled with evaporative lightscattering detection (ELSD) [13]. However, few studies havebeen focus on Bulbus Fritillariae Thunbergii. In this paper,high-speed counter-current chromatography is coupled with evapora-tive light scattering detection for separation and purification ofverticine and verticinone from the extracts of Bulbus FritillariaeThunbergii by a one-step HSCCC separation. 2. Experimental 2.1. Materials Bulbus Fritillariae Thunbergii (Zhejiang, China) was pur-chased from Shanghai Kangqiao Medical Factory, authenticatedby Shanghai Chinese Traditional Medicine Research Instituteand fitted for Chinese Pharmacopoeia. All solvents used for thepreparation of crude extracts and HSCCC separation were of Fig. 1. Chemical structures of verticine and verticinone. analytical grade (Chinese Medication Group Shanghai ChemicalReagent Company, Shanghai, China). Methanol (Merck), ace-tonitrile (Merck) and pure water used for HPLC was treated withSAGA-30D Super Pure Water System (Saga Electronic Tech-nology, Co., Ltd., Shanghai, China). Verticine and verticinonestandard samples were purchased from National Institute for theControl of Pharamaceutical and Biological Products, Ministryof Health, Beijing, China. 2.2. Preparation of crude samples The 100g dried Bulbus Fritillariae Thunbergii was groundto powder (60 mesh) by a disintegrator (Shanghai Experimen-tal Instrument Company, Shanghai, China) and loaded intomicrowave extractor (Model VIP 272, National EngineeringResearch Center for Chinese Traditional Medicine, Shanghai,China) and extracted at 680 W of microwave power in 90%ethanol (with a ratio of the powder to solvent 1:10) for 20 min.The extraction solution was filtered and concentrated to drynessby rotatory evaporator under reduced pressure and dried undervacuum (ZK 82J electrothermal vacuum desiccator, ShanghaiExperimental Instrument Company).The residue was stored ina desiccator. 2.3. Selection oftwo-phase solvent system The selection of the two-phase solvent system for the targetcompounds is the most important step in HSCCC, which mayaccount for 90% of the entire work in HSCCC[14]. Two princi-ples are generally followed. First, a suitable partition coefficient(K), which is the ratio of solute distributed between the mutu-ally equilibrated two solvent phases. The suitable K-value forHSCCC are in the range of 0.5-1.0; second, higher retention ofthe stationary phase normally results in better peak resolution.If the setting time of the two-phase solvent system is less than20s, the solvent system would provide satisfactory retentionof the stationary phase. A two-phase solvent system composedof chloroform-ethanol-0.2 molL-1hydrochloric acid (3:2:2,v/v/v) was chosen for the separation and purification of the crudeextracts. 2.4. High-speed counter-current chromatography (HSCCC) 2.4.1. Instrumentation The preparative HSCCC instrument (Model TBE-300A,Shanghai Tauto Biological Company, China) was equipped withthree preparative coils connected in series (diameter of polyte-trafluoroethylene (PTFE) tube, 2.6 mm; total volume, 119 mL)and a 10 mL sample loop. The revolution speed of the instru-ment is regulated with a speed controller in the range between0 and 999 rpm.Constant temperature circulator (HX-1050, Bei-jing Boyikang Experimental Apparatus Company, China) wasused to control the temperature.The solvent was pumped into thecolumn with the AKTA purifier pump P-900 (Amersham, USA)at a flow rate of up to 10 mL/min and pressure up to 25 MPa.An optimum speed of 1.2 mL/min was used in the experiment.The continuous monitoring of the effluent was achieved withevaporative light scattering detection (Alltech ELSD 2000ES,Alltech,USA). 2.4.2. HSCCC separation procedure The multiplayer coiled column was first fully filled with theupper phase (stationary phase) and lower phase (mobile phase)simultaneously at a flow rate of 10mL/min. Then the lower phasealone was pumped at a flow rate of 1.2 mL/min. In the mean-time, the HSCCC apparatus was rotated at 800 rpm, constanttemperature was 25°C. After 30 min, the lower phase emergedin the effluent and hydrodynamic equilibrium was establishedin the column. A sample solution containing 200 mg BulbusFritillariae Thunbergii extracts in 10 mL of the lower phase ofthe two-phase solvent system was injected through the injec-tion valve. The effluent was continuously monitored by ELSDand each peak fraction was manually collected according to thechromatogram. After using thin layer chromatography (TLC) asa primary comparison with the standard sample of verticine andverticinone, HPLC analysis was carried out. 2.5. HPLC analysis, mass spectrometry and'H NMRidentification of the fractions HPLC analysis was performed with an Agilent/HP 1100series (Agilent, USA) equipped with an Alltech ELSD 2000ESdetector (Alltech USA). The Agilent/HP 1100 series HPLCwas consisted of a vacuum degasser, quandary pump, ther-mostated column compartment, diode array detection and injec-tion valve with a 20 pL loop. The crude extracts of BulbusFritillariae Thunbergii and each purified peak fraction fromthe preparative HSCCC separation were analyzed by HPLC(Agilent Exlipse XBD C1: column, 5 um,4.6 mm×150mm),eluted with acetonitrile-water-diethylamine (70:30:0.3, v/v/v)at a flow rate of 1.0 mL/min, and column temperature was 30°C.ELSD parameters were as follows: drift tube temperature,90°C;gas flow, 2.4 L min-1; impactor, off. The purified fractions ofverticine and verticinone obtained from the preparative HSCCCseparation were analyzed by electron impact mass spectroscopy(EI-MS) (GC-TOFMS, Micromass, UK) and lHNMR (BrukerAdvance 500MHz spectrometer referenced to tetramethylsi-lane), respectively. Please cite this article as: Zhilan Liu et al., Separation and purification of verticine and verticinone from Bulbus Fritillariae Thunbergii by high-speed counter-current chromatography coupled with evaporative light scattering detection, Talanta (2006), doi:10.1016/j.talanta.2006.08.023 Table 1The K-values of verticine and verticinone in different two-phase solvent systems Solvent system K-value Verticinone Verticine n-Butanol-ethyl acetate-water (2:3:5,v/v/v) 18 15 n-Butanol-ethyl acetate-water (1:2:3,v/v/v) 16 14 n-Butanol-ethyl acetate-water (2:1:3, v/v/v) 16 15 n-Hexane-diethyl ether-ethanol-water 1.68 3 (1:5:2:5,v/v/v/v) Chloroform-ethanol-water (4:2:2, v/v/v) 1.67 2.06 Chloroform-ethanol-0.2molL- 0.98 1.02 hydrochloric acid (2:3:2, v/v/v) Chloroform-ethanol-0.2molL- hydrochloric acid (3:2:2, v/v/v) 0.87 1.16 Experimental procedure: 5 mL of each phase of the pre-equilibrated two-phasesolvent system was poured in a 20 mL test tube and 10 mg of the sample wasadded. The tube was ultrasonic for 2 min and waited the solvent to separatecompletely. A 100 pL of each layer was taken out and evaporated. The residuewas dissolved in 1 mL methanol and analyzed by HPLC for the K-value. TheK-value was expressed as the peak area of target compound in the upper phasevs. in the lower phase. 3. Results and discussion 3.1. HSCCC separation ofverticine and verticinone fromthe crude extract In the HSCCC experiment, a good solvent system can pro-vide an ideal partition coefficient (K) for the target compounds.The most suitable K-value of the target compounds is closeto 1. In our experiment, two compounds want to be separatedby one solvent system, so they must have different K-values.According to solubility of verticine and verticinone, preliminaryHSCCC studies were carried out with seven different two-phasesolvent systems, such as n-butanol-ethyl acetate-water (2:3:5,1:2:3, 2:1:3, v/v/v), n-hexane-diethyl ether-ethanol-water(1:5:2:5, v/v/v/v), chloroform-ethanol-water (4:2:2, v/v/v)and chloroform-ethanol-0.2molL-hydrochloric acid (2:3:2,3:2:2, v/v/v). The measured K-values are shown in Table 1.The two-phase solvent systems of n-butanol-ethyl acetate-water Fig. 2. Chromatogram of crude extracts of Bulbus Fritillariae Thunbergiiby HSCCC. Peak 3, verticinone; peak 5, verticine. Experimental condition:two-phase solvent system; chloroform-ethanol-0.2 molL- hydrochloric acid(3:2:2, v/v/v); stationary phase, upper phase; mobile phase, lower phase; flow-rate, 1.2 mL/min; rotary speed, 800 rpm;ELSD condition: drift tube temperature,64.8C; gas flow, 2.1 L min-1; impactor: off, sample size, 200 mg of crudeextracts dissolved in 10 mL of the lower phase; retention of the stationary phase,0.58; separation temperature,25°C. and n-hexane-diethyl ether-ethanol-water did not give effec-tive separation. The chloroform-ethanol-water system gavegood result, however, it also led to emulsification. In the sub-sequent studies, this system was replaced with chloroform-ethanol-0.2 molL- hydrochloric acid. After comparison ofdifferent ratios, chloroform-ethanol-0.2 molL- hydrochloricacid (3:2:2, v/v/v) gave the best separation. Other factors such as the revolution speed of the separationcolumn, the flow rate of the mobile phase and the separation tem-perature, were also investigated. Emulsification seemed to occurwhen the revolution speed is higher than 800 rpm. Ultimately,a flow rate of 1.2 mL/min, a revolution speed of 800 rpm andseparation temperature of 25C gave the best separation. Fig. 2showed the preparative HSCCC separation chromatogram. A25.6 mg of verticine and 10.3 mg of verticinone were obtainedfrom the 200 mg crude extracts. 3.2. HPLC analysis The method of HPLC analysis was referenced in ChinesePharmacopoeia (2005). The crude samples and peak fractionsseparated by HSCCC were analyzed by HPLC under the ana-lytical conditions described in Section 2.5. The chromatograms Fig. 3. HPLC chromatogram of crude extracts of Bulbus Fritillariae Thunbergi (A), peak 5 from preparative HSCCC (B), peak 3 from preparative HSCCC (C)and the standard sample of verticine and verticinone (D). Experimental condition: Aglient Exlipse XBD C18 column (5 pm, 4.6mmx150mm); mobile phase,acetonitrile-water-diethylamine (70:30:0.3); flow rate, 1.0 mL/min; temperature, 30°C; ELSD condition: drift tube temperature, 90°C; gas flow, 2.4L min;impactor, off. Please cite this article as: Zhilan Liu et al., Separation and purification of verticine and verticinone from Bulbus Fritillariae Thunbergii by high-speed counter-current chromatography coupled with evaporative light scattering detection, Talanta (2006), doi:10.1016/j.talanta.2006.08.023 were shown in Fig. 3. The purified samples of verticine and ver-ticinone calculations were made by comparison of the peak areawith the standard. The results showed that peak 3 correspondedto verticinone and peak 5 corresponded to verticine, and theirpurity were 95.4 and 96.8%, respectively. 3.3. Structural identification The identities of peaks 3 and5 in HSCCC were determined byelectron impact mass spectroscopy (EI-MS) and HNMR. Peak3HNMR (CDCl3) 8 (ppm): 0.78 (s, 3H, 19-CH3), 1.02 (s,3H,21-CH3), 1.08 (d,J=7.0Hz,3H,27-CH3), 3.58(m, 1H,3-CH);EI-MS: m/z, 429 (M*), 414,384,372,154,124, 112(100%).Peak 5 1HNMR (CDCl3) 8 (ppm): 0.82 (s, 3H, 19-CH3), 1.05(s,3H,21-CH3), 1.10 (d,J=7.1 Hz, 3H,27-CH3), 1.25 (m,1H,5-CH), 3.60 (m, 1H, 3-CH); EI-MS: m/z, 431 (M+),412,386,154, 112 (100%). Compared with the data given in reference[15], the compounds in peaks 3 and 5 were verticinone andverticine, respectively. 4. Conclusion The overall results of our studies indicated that HSCCC cou-pled with evaporative light scattering detection was successfullyused for separation and purification of verticine and vertici-none from Bulbus Fritillariae Thunbergii. The present studyalso demonstrated that HSCCC coupled withELSD is a powerful tool to monitor separation and purification of non-chromophoricactive substances from natural medicines. Acknowledgements Financial supports from Shanghai Commission of Scienceand Technology and National Engineering Research Center forTraditional Chinese Medicine are gratefully acknowledged. ( References ) ( [1] P harmacopoeia of the People’s Republic of China, the first division of 2005 e dition, China Chemical Industry Press, Beijing, 2005, p.205. ) ( [2] Z.J. Shang, X.L. L iu, Chin. J. Med. H ist. 25 (1995) 38. ) ( [3] D.M. Xu, Y.J. Xu, Chin. Trad. H e rbal Drugs 22 (1991) 132 . ) ( [4] G. L in, Y.P. Ho, P. Li, X.G. Li,J. Nat. Prod. 58 (1995) 1 662. ) ( [5] P . Li, G.J. Xu, L.S. Xu, Y.X. Wang, Phytother. Res. 9 ( 1 995) 460. ) ( [6] P . Li, X.G. L i, G.J. Xu, J .Chin. P h arm. Un i v. 21 (199 0 ) 198. ) ( [7] P. Li, H. Ji, S. Zhou, Chin. Trad. Herbal Drugs 24 ( 1 993) 475. ) ( [8] J.X. Zhang, G.E. Ma, A.N. Lao, R .S. Xu, Acta P harm. S in. 26 (1991) 231. ) ( [9] F.Q. Yang, T.Y. Zhang, R. Zhang, Y. Ito, J. Chromatogr. A 829 (1 9 98) 137. ) ( [ 1 0] X.L. Cao, Y. Tian, T.Y. Z hang, X. Li, Y. Ito, J. Chromatogr. A 855 (1999)709. ) ( . [11] T.H. Huang, P.N. Shen, Y.G. Shen, J. Chromatogr. A 1 066 (2005) 239. ) ( [12] J.H. Chen,F.G. Wang,F.S.C. Lee,X.R. Wang, M.Y. Xie, T a lanta 69 (2006) 172. ) ( [ 1 3] X. Cao, Y . Ito, J. Chromatogr. A 1021 (2003) 1 17. ) ( [ 1 4] Y . Ito, J. Chromatogr. A 1065 (2005)145. ) ( [15] K . Kanwho,M. Tanaka, K. Haruki, Ch e m. Pha r m. Bull. 28 (1980) 134 5 )
关闭-
1/4

-
2/4
还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
上海同田生物技术有限公司-高速逆流色谱仪HSCCC为您提供《浙贝母中分离纯化贝母碱和去氢贝母碱检测方案(高速逆流色谱)》,该方案主要用于中药材和饮片中含量测定检测,参考标准《暂无》,《浙贝母中分离纯化贝母碱和去氢贝母碱检测方案(高速逆流色谱)》用到的仪器有TBE-1000A制备色谱仪/萃取仪/快速分离制备色谱仪、TBE-200V 高速逆流色谱仪、TBE300B+AKTA高速逆流色谱仪/离心分配色谱/萃取仪/制备色谱仪。
我要纠错
推荐专场
相关方案







 咨询
咨询