方案详情文
智能文字提取功能测试中
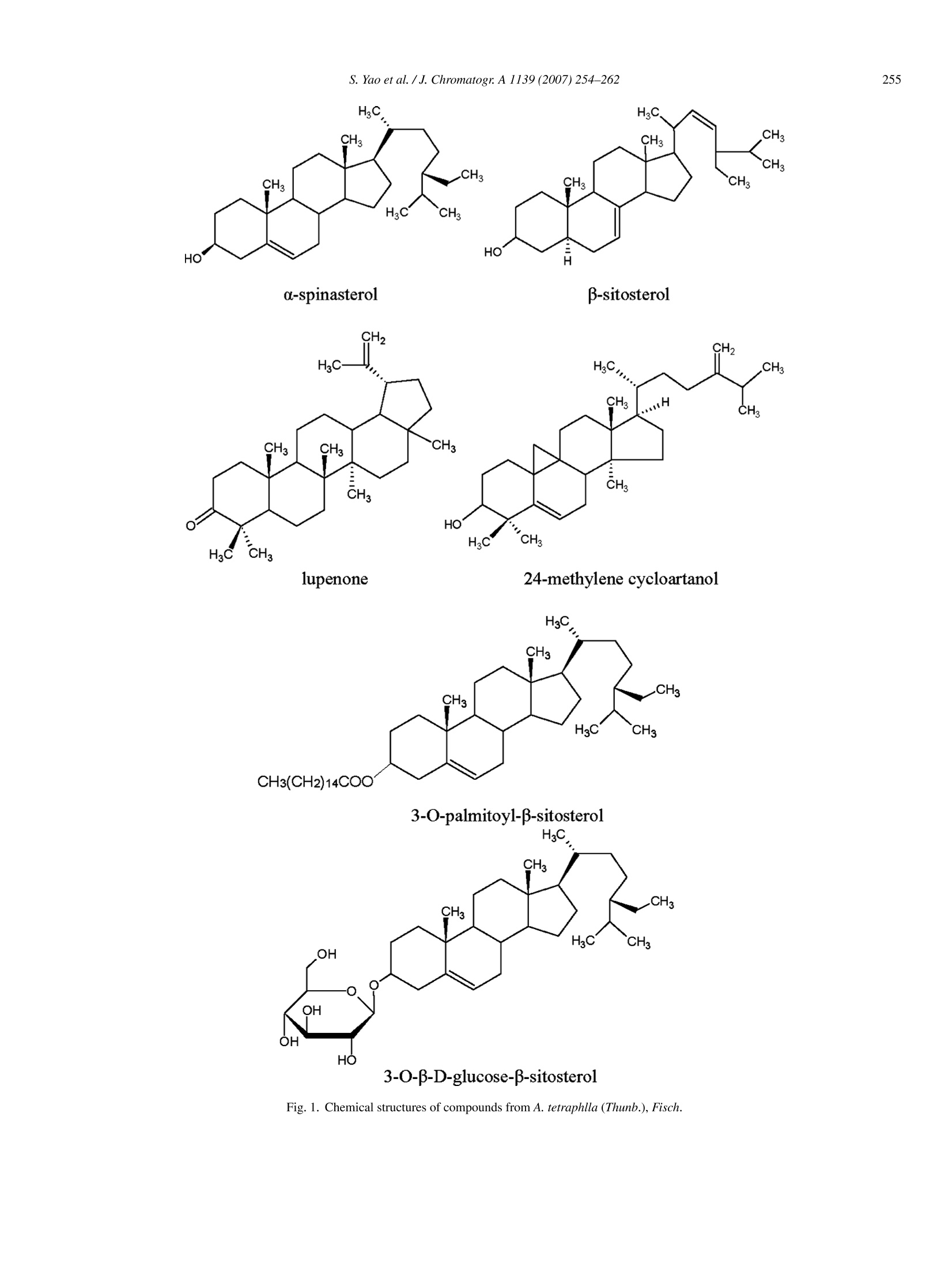
Available online at www.sciencedirect.comScienceDirectJOURNAL OFCHROMATOGRAPHY AJournal of Chromatography A, 1139 (2007) 254-262 S. Yao et al. /J. Chromatogr. A 1139 (2007)254-262 www.elsevier.com/locate/chroma Preparative isolation and purification of chemical constituents fromthe root of Adenophora tetraphlla by high-speed counter-currentchromatography with evaporative light scattering detection Shun Yao, Renming Liu a,b, Xuefeng Huang , Lingyi Kong a,* Department ofNatural Medicinal Chemistry, China Pharmaceutical University, Nanjing 210009, China College of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252059, China Received 29 July 2006; received in revised form 6 November 2006; accepted 10 November 2006Available online 5 December 2006 Abstract Preparative high-speed counter-current chromatography (HSCCC), as a continuous liquid-liquid partition chromatography with no solid supportmatrix,combined with evaporative light scattering detection (ELSD) was employed for systematic separation and purification of non-chromophoricchemical components from Chinese medicinal herb Adenophora tetraphlla (Thunb.), Fisch. Nine compounds, including a-spinasterol, B-sitosterol,nonacosan-10-ol, 24-methylene cycloartanol,lupenone, 3-O-palmitoyl-B-sitosterol,3-O-B-D-glucose-B-sitosterol, eicosanoic acid and an unknowncompound, were obtained. The compounds were all above 95% determined by high-performance liquid chromatography (HPLC)-ELSD, and theirstructures were identified by HNMR and chemical ionization mass spectroscopy (CI-MS). The results demonstrate that HSCCC coupled withELSD is a feasible and efficient technique for systematic isolation of non-chromophoric components from traditional medicinal herbs. @ 2006 Elsevier B.V. All rights reserved. Keywords: High-speed counter-current chromatography; Evaporative light scattering detection; Adenophora tetraphlla (Thunb.),Fisch; Systematic isolation andpurification 1.Introduction High-speed counter-current chromatography (HSCCC) is akind of support-free all-liquid partition chromatography thatwas first invented by Ito [1]. As no solid stationary phase isused, it eliminates irreversible adsorption of sample onto thesolid support used in the conventional chromatographic col-umn. What is more, it has a large amount of sample injection;multiform relative pure substances can be obtained at one time It is well recognized that evaporative light scattering detec-in large amount. So it is especially suitable for separation and tion (ELSD) can outperform traditional detection methods whenpurification of active components from natural products. As an analyzing samples having no or end ultraviolet absorption byadvanced separation technique, HSCCC has been widely used high-performance liquid chromatography (HPLC). Analyticalfor separation of active components from traditional Chinese HSCCC coupled with ELSD had been reported previouslyherbs and other natural products in recent years. Successful [16,17]. Preparative HSCCC combined with ELSD was alsoapplication of HSCCC has been reported for the purification reported [18]. A split valve was used to connect the prepara-of alkaloids [2-4], quinones [5-7], flavonoids [8,9], coumarins tive HSCCC and ELSD system. But the split parameters and the[10,11], other natural products [12-14] and so on. For these ELSD condition were not given, and the purity of the obtainedcompounds was also determined with a UV detector at 204 nm.The two-phase solvent system used also contained CHCl3 whichis deleterious to humans and environment. In the present paper,diverse methods of preparative HSCCC coupled with ELSD ( * Corresponding author. Tel.:+86 25 85391289; fax: +86 25 85301528. E-mail address: ly k ong @jlon l ine.com (L. Kong).0021-9673/$-see front matter C 2006 Elsevier B.V. All rights reserved. doi:10 . 1016/j.chroma.2006. 1 1.056 ) chromophoric constituents, UV detector has become the majordetection instrument of HSCCC to monitor the column effluent[15]. But its application to HSCCC is limited by its inherentshortcomings. It cannot be used as the detector for separationof non-chromophoric components and makes the application ofHSCCC restricted to some degree. So the study on the applica-tion ofother kind of detector for HSCCC separation is of greatinterest. H3C, HC, CH3 CH3 CH3 CH CH3 CH3 CH, CH, H3C CH HO HO H a-spinasterol B-sitosterol lupenone 24-methylene cycloartano1 H3C Fig. 1. Chemical structures of compounds from A. tetraphlla (Thunb.),Fisch. Fig. 1. (Continued). were established for systematic separation and purification ofchemical components from different fractions of Chinese medic-inal herb Adenophora tetraphlla (Thunb.), Fisch, which is listedin China Pharmacopoeia as an antiinflammatory and antitussivedrug used in the treatment of lung disease [19]. Nine compoundswithnon-chromophoric absorption were obtained. The chemicalstructures of the compounds are given in Fig. 1. 2. Experimental 2.1. Apparatus The HSCCC instrument employed in the present study wasa TBE-300 high-speed counter-current chromatography (TautoBiotechnique, Shanghai, China) with three multilayer coil sepa-ration columns connected in series (I.D. of the tubing=1.5 mm,total volume=300 ml) and a 20 ml sample loop. The revolutionradius was 5 cm, and the values β (B=r/R, where r is the rotationradius or the distance from the coil to the holder shaft, and R is therevolution radius or the distance between the holder axis and cen-tral axis of the centrifuge) of the multilayer coil varied from 0.5at internal terminal to 0.8 at the external terminal.The revolutionspeed of the apparatus can be regulated with a speed controllerin the range between 0 and 1000 rpm. The system was alsoequipped with one S-1007 constant flow pump (Shenyitong Tech& Exploitation, Beijing,China), an Alltech 2000ES ELSD (All-tech, USA). The connection of the system is shown in Fig. 2. Atee junction valve was used to connect the outlet of HSCCC col-umn with the inlet of the ELSD system and pipeline of fraction collector. The splitflow of the effluent was adjusted by adjustingthe valve, and half of the effluent from the outlet of the col-umn was monitored by ELSD. The data were collected with themodel N2000 chromatography workstation (Zhejiang Univer-sity, Hangzhou, China). The HPLC equipment used was Agilent1100 series system and Agilent HPLC workstation (Agilent,USA). Nuclear magnetic resonance (NMR) spectrometer usedhere was Bruker AM-500 MHz (Bruker, Switzerland). Chemi-cal ionization mass spectroscopy (CI-MS) used was ShimadzuGC-MS 2010QP (Shimadzu,Japan). 2.2. Reagents All solvents used for HSCCC were of analytical grade (Han-bon Sci & Tech,Jiangsu, China). Methanol and acetonitrile usedfor HPLC were of chromatographic grade (Hanbon Sci & Tech);D-101 macroporous resin (Chemical Plant of Nankai University,Tianjin, China) was used for sample purification and the waterused was distilled water. The dried roots of A. tetraphlla (Thunb.), Fisch were pur-chased from a local drug store and identified by ProfessorMingjian Qing, Department of Medicinal Plant, China Phar-maceutical University. 2.3. Preparation ofcrude extract Eight hundred grams dried roots of A. tetraphlla (Thunb.),Fisch was powdered and extracted by 81 90% ethanol, and theextract was evaporated to form a syrup. The syrup was then Fig. 2. Roadmap of extraction and separation. Solvent system 1: n-hexane-ethyl acetate-acetonnitrile 5:1:5 (v/v/v); solvent system 2: n-hexane-ethylacetate-acetonnitrile 5:1:4 (v/v/v); solvent system 3: n-hexane-ethyl acetate-acetonnitrile 5:2:5 (v/v/v); solvent system 4: light petroleum (b.p.60-90°C)-ethylacetate-ethanol-water 6:4:5:5(v/v/v/v). dissolved in 1.5 l water by sonication and partitioned with etherand n-butanol of equal volume three times successively. Bothether and n-butanol solution were vacuum evaporated at 65°C.About 20 g residue of ether and 18 g residue of n-butanol wereobtained, respectively. In order to enrich the target componentsand remove impurities, the residue of n-butanol was loaded onD-101 macroporous resin column (35 cm ×3.4 cm, the volumeof column was 170 ml) and eluted with 1.71 distilled waterand 1.51 75% ethanol, respectively. The 75% ethanol effluentwas collected and evaporated at 65°C in vacuum and about810 mg residues were obtained. All the residues were stored ina refrigerator (5°C) for further use. 2.4. Preparation oftwo-phase solvent system and samplesolution In the present study, the two-phase solvent system composedof n-hexane-ethyl acetate-acetonnitrile 5:1:5 (v/v/v), 5:1:4(v/v/v), 5:2:5 (v/v/v) and light petroleum (b.p.60-90°C)-ethylacetate-ethanol-water 6:4:5:5 (v/v/v/v) were used for HSCCC separation. Each component of the solvent system was addedto a separatory funnel and thoroughly equilibrated at roomtemperature for 12h. The upper phase and lower phasewere separated and degassed by sonication for 30 min shortlybefore use. For HSCCC separation of ether extracts, the sample solutionwas prepared by dissolving 360 mg of ether extract in 3 ml ofthe upper phase and 3 ml of the lower phase of n-hexane-ethylacetate-acetonnitrile 5:1:5 (v/v/v), and seriously filtering withmicropore membrane filters (d=0.45 um). The sample solu-tion for HSCCC separation of refined n-butanol fraction wasprepared by dissolving 400 mg of refined n-buthanol fraction in5 ml of the lower phase of light petroleum (b.p.60-90°C)-ethylacetate-ethanol-water 6:4:5:5 (v/v/v/v) and seriously filteringwith micropore membrane filters (=0.45 um). 2.5. HSCCC separation The HSCCC system was connected according to Fig. 2. Ineach separation process, the coiled column was first entirely Fig. 3. Schematic diagram of the HSCCC-ELSD system and design of T-split. Fig. 4. HSCCC chromatograms of two fractions of ether (A-C),n-butanol (D)from A. tetraphlla (Thunb.), Fisch. Conditions of (A) two-phase solvent system:n-hexane-ethyl acetate-acetonnitrile 5:1:5 (v/v/v); stationary phase: upperorganic phase; mobile phase: lower phase; flow rate: 1.5 ml/min; revolutionspeed: 800 rpm; sample size: 360 mg of ether extracts dissolved in 3 ml of theupper phase and 3 ml of lower phase; retention of the stationary phase: 59%.Con-ditions of (B) two-phase solvent system: n-hexane-ethyl acetate-acetonnitrile5:1:4 (v/v/v), mobile phase: lower aqueous; flow rate: 2.0 ml/min; revolutionspeed: 800 rpm; retention of the stationary phase: 69%. Conditions of (C) filled with the upper organic stationary. Then the apparatus wasrotated at 800 rpm, while the lower aqueous mobile phase waspumped into the column at a flow rate of 1.5 ml/min for pre-liminary separation and 2.0 ml/min for further separation. Afterthe mobile phase front emerged and the system establisheda steady state hydrodynamic equilibrium, the sample solutionwas injected into the separation column through the injectionvalve. The split-flow of the effluent was adjusted by adjust-ing the switch valve, and half of the effluent from the outletof the column was monitored by ELSD. The tube temperatureof ELSD was 110°C, and gas flow rate was set at 1.5 l/min inthe above course of detection. The whole process of separa-tion was carried out under room temperature (22.0-25.5°C).Each peak fraction was manually collected according to thechromatogram and concentrated under reduced pressure. Theresiduals were dissolved in methanol for subsequent HPLCanalysis. 2.6. HPLC analysis and identification ofHSCCC peakfractions The HPLC analysis of every fraction of A. tetraphlla(Thunb.), Fisch crudee extract and HSCCC peak1frac-tion was performed with a Shim-Pack CLC-ODS column,(150mmx4.6mm I.D., 5um) at room temperature. Foranalysis of the fraction of ether, the mobile phase wasacetonnitrile-water (98:2, v/v) and the flow rate was set at1.0 ml/min constantly. When the refined n-buthanol fraction wasanalyzed, the mobile phase was methanol and water in gradientmode as follows: 5:95-100:0 in 60 min, and the flow rate waskept at 1.0 ml/min constantly. Tube temperature of ELSD was110°C, and gas flow was set at 1.5 1/min in the above analysissame as HSCCC separation process. 3. Results and discussion In the present study, D-101 macroporous resin was used topurify crude extract of n-butanol fraction. Water was first usedto remove plentiful hydrosoluble chemicals such as pigmentsand polysaccharides, which had no or little retention on D-101 macroporous resin. Secondly, 75% ethanol was used toelute most target compounds, which was prepared for furtherHSCCC isolation and purification. At last, 95% ethanol wasused to activate the resin for another use. Consequently, 810 mgrefined n-butanol fraction was obtained from 800 g A. tetraphlla(Thunb.), Fisch, using this purification process. A road map ( two-phase s o lvent s y stem: n-hexane-ethyl ace t ate-acetonnitrile 5:2 : 5 (v/ v /v), m obile phase:l o wer aqueous; flow rate: 2.0 ml/min; revolution speed: 800 rpm; retention of the stationar y phase : 66%. Conditions of (D) two-phase s olvent system: l ight petroleum (b.p. 60-90°C)-ethyl ac e tate-ethanol-water 6:4:5:5(v/v/v/v); mobile phase: the lower phase; flow rate: 1. 5 ml/min; revolution speed:800 rpm; sample size: 400 mg of refined n-buthanol extract in 5 ml of the lowerphase, r etention of the stationary phase: 6 1%. E L SD condition: tube temper- ature was 1 10° C , gas flow was 1.5 1/min. A ll the samples f or HSCCC were filtered with micropore m embrane filters (D=0.45 um) strictly, and se p aration temperature was all under room temperature (22.0-25.5°C). ) of the whole work is given in Fig. 3, and the HSCCC chro-matograms are shown in Fig. 4. 3.1. HPLC analysis and identification ofHSCCC peakfractions The ether fraction, refined n-butanol fraction and each peakfraction of HSCCC was analyzed by HPLC. On the basis of theresearch of refs. [20,21], good HPLC conditions were inves-tigated in this research. Isocratic and gradient elution wereemployed to obtain optimum resolution equally. According tothe adopted methods, the purities of the eight compounds wereall above 95%, and their HPLC chromatograms and correspond-ing purity were shown in Fig. 5. Identification of the HSCCCpeak fractions was based on retention time together with HNMR and CI-MS. 3.2. Selection oftwo-phase solvent system and otherconditions ofHSCCC Successful separation by HSCCC largely depends upon theselection of suitable two-phase solvent system. As for the con-stituents mainly composed of sterols, their separation is not aneasy task. In this experiment, several kinds of solvent systemswere tested. The ideal K-values of target compounds should be ina proper range. In general, small K-values usually result in poorpeak resolution, while large K-values tend to produce excessivesample band broadening. For separation of sterols, which were the main constituents ofether fraction, n-heptane-ethyl acetate-acetonnitrile was neveremployed before[22]. Because the polarity of target com-pounds is too low, perfect K-value cannot be gained in thesolvent systems containing water. Considering that n-hexane Fig. 5. HPLC chromatograms of two fractions of ether (A) and n-butanol (H) from A. tetraphlla (Thunb.), Fisch and HSCCC peak fractions of them (B-G,H-K).Column: Shimadzu VP-ODS (150 mmx4.6mm I.D., 5 pm); mobile phase (A-G): acetonnitrile-water (98:2, v/v); flow rate: 1.0 ml/min; mobile phase (H-K):methanol-water (methanol: 5-100% in 60 min); flow rate: 1.0 ml/min; ELSD tube temperature: 110°C, gas flow rate: 1.51/min. Fig. 5. (Continued). was more inexpensive, it was advisable to substitute n-heptanewith n-hexane from the point of view of mass production. So n-hexane-ethyl acetate-acetonnitrile was chosen as the two-phasesolvent system for HSCCC separation of ether fraction. The K-values of the target constituents in several solvent systems areshown in Table 1. From Table 1, it can be seen that for separa-tion of ether fraction, whenn-hexane-ethylacetate-acetonnitrile(5:1:4, v/v/v) was used as the solvent system, compounds Iand II could be separated well, but the K-values of com-pounds III, IV, V and VI were too high. When n-hexane-ethylacetate-acetonnitrile (5:1:5,v/v/v) was used as the solvent sys-tem, the K-values of compounds I and II were too close, and thatof V and VI too high. But in this system, compounds III and IVcould be separated. When n-hexane-ethyl acetate-acetonnitrile(5:2:5, v/v/v) was used as the solvent system, compounds V andVI could be separated well. So the ether fraction was first sep- arated with n-hexane-ethyl acetate-acetonnitrile (5:1:5,v/v/v).The HSCCC chromatogram is shown in Fig. 4(A). During thisseparation process, four peaks (I, II, III and IV in Fig. 4) werecollected. Peaks III and IV were approved to be nonacosan-10-o1and 24-methylene cycloartanol. Peaks I and II were too close andthe purities of I and II were not satisfactory, so peaks I and II werecollected together and further separated with n-hexane-ethylacetate-acetonnitrile (5:1:4,v/v/v). The HSCCC chromatogramis shown in Fig. 4(B). Peak I was identified as a-spinasterol andpeak II as B-sitosterol. The compounds with large K-values werestill maintained in the separation column. So the residual solu-tion in the separation column was blown out with compressednitrogen gas after the four major peaks were obtained. Then theresidual solution was concentrated to dryness and separated withn-hexane-ethyl acetate-acetonnitrile (5:2:5, v/v/v) as the sol-vent system. The HSCCC chromatogram is shown in Fig. 4(C).At last, another two compounds (peaks V and VI) were obtainedand identified as lupenone and 3-O-palmitoyl-B-sitosterol. Enlightened by Ito’s strategy of solvent system selection [23],light petroleum (b.p. 60-90°C)-ethyl acetate-ethanol-water5:5:5:5 (v/v/v/v) was tried for separation of the refined n-buthanol fraction at first. But it was given up quickly forpoor retention of the stationary phase and resolution. So lightpetroleum (b.p. 60-90°C)-ethyl acetate-ethanol-water 6:4:5:5(v/v/v/v) was used as the two-phase solvent system. The upperorganic phase was used as the stationary phase and the loweraqueous phase as the mobile phase. Three compounds includingan unknown compound, eicosanoic acid and 3-O-B-D-glucose-B-sitosterol (peaks VIII, IX and X in Fig. 4), were acquiredfrom refined n-buthanol fraction. The HSCCC chromatogram isshown in Fig. 4(D). 3.3. The structural identification The structural identification of peak fractions was performedwith CI-MS and lHNMR, with TMS as internal standard. Dataof each compound were given as follows. DataofHSCCC peakI (6.3 mg):CI-MS((m/z):413([M+H]*); H NMR (500MHz, CDCl3): 5.16 (1H,s), 5.13(1H, dd), 5.02 (1H, dd), 3.58 (1H, m), 1.03 (3H, d,J=6.6 Hz), 0.87 (3H, t, J=8.9Hz), 0.82 (6H, d,J=8.0Hz),0.79 (3H, s),0.55(3H, s). The results were very similar to thosein ref. [24], peak I corresponded to a-spinasterol. Data of HSCCC peak ⅡI (9.7mg): CI-MS(m/z): 415([M+H]t); H NMR (500MHz, CDCl3): 5.40 (1H, br d),3.58(1H,m),0.69-1.01(CH3×6). The results were very similarto those in ref. [25], peak II corresponded to B-sitosterol. Data of HSCCC peak ⅢI (2.9mg): CI-MS (m/z): 425([M+H]+);; H NMR (500MHz, CDCl3): 3.63 (1H, m),1.44-1.24 (m, 23H), 0.89 (6H, t, J=7.1Hz, CH3×2). Theresults were very similar to those in ref. [26],peak II corre-sponded to nonacosan-10-ol. Data of HSCCC peak IV (3.1mg): CI-MS (m/z): 441([M+H]*); H NMR (500MHz, CDCl3): 4.72, 4.67 (2H, d,J=2.7 Hz), 3.21 (1H, m), 1.35 (6H, d,J=7.0Hz), 1.07, 1.06,0.99,0.91,0.87 (s, CH3×5), 0.96 (3H, d,J=5.1Hz), 0.57 (1H,d,J=4.5Hz),0.34(1H,d,J=4.5Hz). The results were very sim- Table 1The K-values of target components measured in different solvent systems Solvent system K-value n-Hexane-ethyl n-Hexane-ethyl n-Hexane-ethyl Petroleum ether-ethyl acetate-acetonnitrile acetate-acetonnitrile acetate-acetonnitrile acetate-ethanol-water (5:1:4, v/v/v) (5:1:5, v/v/v) (5:2:5, v/v/v) (6:4:5:5,v/v/v/v) I 2.11 1.07 2.49 1.12 Ether II >5 1.41 一 fraction IV 一 1.63 V - >5 2.93 VI - 4.27 一 Refined n-butanol fraction VII - — 0.85 VIII - 1.22 IX 1.45 Experimental protocol: 4 ml of each phase of the equilibrated two-phase solvent system was added to approximately 8 mg of crude sample placed in a 10 ml testtube. The test tube was caped and shaken vigorously for 2 min to equilibrate the sample thoroughly. An equal volume of each phase was then analyzed by HPLC toobtain the partition coefficient (K). The partition coefficient (K) value was expressed as the peak area of the compound in the upper phase divided by the peak areaof the compound in the lower phase. ilar to those in ref. [27], peak IV corresponded to 24-methylenecycloartanol. Data of HSCCC peak.kV(5.2mg): CI-MS (m/z): 425([M+H]*);HNMR (500MHz, CDCl3): 4.57, 4.68 (1H each,m), 2.42 (2H,m), 0.80-1.68 (CH3×6,s). The results were verysimilar to those in ref. [25], peak V corresponded to lupenone. Data of HSCCC peak VI (5.7mg): CI-MS (m/z): 653([M+H]+),397 ([M+H-162]+);HNMR(500MHz, CD3OD):5.56 (1H, br), 4.60 (1H, br), 2.30 (2H, t, J=6.5 Hz), 1.25-1.83(55H, m), 0.67-1.14(CH3×7). The results were very similarto those in ref. [28], peak VI corresponded to 3-O-palmitoyl-B-sitosterol. Data of HSCCC peak VII (6.9mg) showed the CI-MS quasi-molecular ion ([M+H]*) peak (m/z: 283), its further structuralidentification will be continued. Data of HSCCC peak VIII (4.5mg): CI-MS (m/z): 313([M+H]*); 1H NMR (500MHz, CDCl3): 0.86 (3H, t, CH3),1.27 (32H, m, CH2×16),1.64(2H, q), 2.35 (2H, t), whichmatched with the reported the data given in ref. [29], peak VIIIcorresponded to eicosanoic acid. Data of HSCCC peak IX (10.2mg): CI-MS (m/z): 577([M+H]*), 415 ([M+H-162]*).Compared with NMR data ofpeak II, there is a group of glucose protons at 8 3.8-5.2 ppm inaddition. The results were very similar to those in ref. [26], peakIX corresponded to 3-0-B-D-glucose-B-sitosterol. 4. Conclusions Some of the chemical constituents from the different frac-tions of A. tetraphlla (Thunb.), Fisch, one of the most popularChinese medicinal herbs, were isolated and purified system-atically by HSCCC. The results of the research provided asuccessful method for isolation of chemical compounds hav-ing no or end ultraviolet absorption with the help of ELSD online. The introduction of online ELSD with HSCCC has dra-matically extended the field of this technique by overcomingthe drawbacks of UV detector. The effluent from the outlet of HSCCC was splitted into two parts: one was collected, while theother was introduced directly into ELSD. The present study indi-cates that this new strategy, which integrates all the advantagesof HSCCC and ELSD, is a very powerful technique possess-ing general utility in the preparative separation and purificationof all kinds of bioactive components from traditional Chinesemedicine (TCM), leaving alone their chromophoric property. Acknowledgement This research work was supported by the National Scien-tific Foundation of China for Outstanding Young Scientists (No.30525032). ( References ) ( [ 1 ] Y. Ito,J. Chromatogr. 214 ( 1 981) 1 22. ) ( [2] R.M. Liu, X. Chu, A . L. S u n, L.Y. Kong, J. Chromatogr. A 107 4 (2005)139. ) ( [3] G. Bringmann, F . T eltschik, M. Michel, S. Busemann, M . R i ckert, R.Haller, S. Bar, S.A. Robertson, R. Kaminsky, Phytochemistry 52 (1 9 99)321 . ) ( [4] F.Q. Yang, Y. Ito, J. Chromatogr. A 943 (2002) 2 1 9. ) ( [ 5] H .B. L i, F. Chen, J. Chromatogr. A 925 ( 2001) 1 09. ) ( [6] H.T. Lu, Y. Jiang, F. Chen, J.Chromatogr. A 1023 (2004) 159 . ) ( [7] R.M. Liu, A .F. Li, A.L. Sun, J. Chromatogr.A 1 0 52 (2004)217. ) ( [8] Q.Z.D u , Z.H. Li, Y. Ito, J. Chromatogr. A 923 (2001) 271. ) ( [9] L.J. Chen,H. Song, X.Q. Lan,D.E. Games,I.A. Sutherland,J. Chromatogr.A 1063 (2005) 241. ) ( [ 1 0] Y . Wei, T .Y. Zhang, Y. Ito, J. Chromatogr. A 1 0 33 ( 2 004) 373. ) ( [11] F .Q. Yang, T.Y. Zhang, Q.H. Liu, G.Q. Xua, Y.B. Zhang,S. Zhang, Y. Ito, J. Chromatogr. A 883 (2000) 67. ) ( [ 1 2] H .T.Lu, Y. Jiang, F . Chen, J. Chromatogr. A 1 026 (2004) 185. ) ( [13] Q .Z. Du, G. Jerz, P. Winterhalter, J. Chromatogr. A 984 (2003) 147. ) ( [14] R. Tsao, R. Yang, J . Chromatogr. A 1 112 ( 2006) 2 02. ) ( [15] D .E. Schaufelberger, T . G. Mccloud, J. Liq. Chromatogr. 12 ( 1989) 2263. ) ( [ 1 6] S . Drogue, M.C. Rolet, D . Thiebaut, R. R o sset, J . Chromatogr. 538 (1991)91. ) ( [ 17] D .E. Schaufelberger, T.G. M c cloud, J. Chromatogr. 538 (1991) 87. ) ( [18] X .L. C a o,T.Y. Zhang, et a l., J . Li q . Chromatogr. Relat. Technol. 26 (20 0 3)1579. ) ( [ 19 ] C hina Pharmacopoeia Committee,Pharmacopoeia of the People’s Republicof China, China Chemical Industry Press, Beijing, 2004,p. 1 7 0 (the first division of 2005 e dition). ) ( [20] P .Breinholder, L . M osca, W. Li n dner, J. Chromatogr. B 777(2002)67. ) ( [21] Q . L i, Z.Y. Tian, T.Z. Jia, Q.T . Xu, Chin. Tradit . Herb. Drugs 36 (2005)1883. ) ( [22] Y.J. Zhou, L.M.Dai, F.M. Cheng, Z.C. Li, Fine Chem. 19 (2002) 18 3 .[23]Y . Ito,J. Chromatogr. A 1 0 65 (2005) 145. ) ( [24] X .Y. Zhao, H . D. Sun, J.Z. Wu, Chin. J. Chin. Ma t er. Med. 30 (2005) 585.[25] S .J. Du, P. Gariboldi, G. Jommi, P lanta M ed. 4 (1986)317. ) ( [26]X.L. Wang, H.X. Lou, J. Chin. P harm. 40 (2005) 1132. ) ( [27] X.F. Sun, S.P . Wang, Z.R. Zheng, J. Chin. Mater . Med. 24 (1999)227. ) ( [28] S .S. Jia, C.M. Ma, L .F. Zhao, A cta Bot. Sin. 32 ( 1990) 468. ) ( [29] Z. Liang, Y.H. Wang, Z.H. L i, H.L. Qin, Nat. Prod. Res.Dev. 1 7 (2005) 409. )
关闭-
1/9

-
2/9
还剩7页未读,是否继续阅读?
继续免费阅读全文产品配置单
上海同田生物技术有限公司-高速逆流色谱仪HSCCC为您提供《南沙参根茎中化学成分检测方案(高速逆流色谱)》,该方案主要用于中药材和饮片中含量测定检测,参考标准《暂无》,《南沙参根茎中化学成分检测方案(高速逆流色谱)》用到的仪器有TBE-200V 高速逆流色谱仪、TBE-20A分析型高速逆流色谱仪/萃取仪/制备色谱仪、TBE300B+AKTA高速逆流色谱仪/离心分配色谱/萃取仪/制备色谱仪、TBE-1000A制备色谱仪/萃取仪/快速分离制备色谱仪。
我要纠错
推荐专场
相关方案








 咨询
咨询