方案详情文
智能文字提取功能测试中
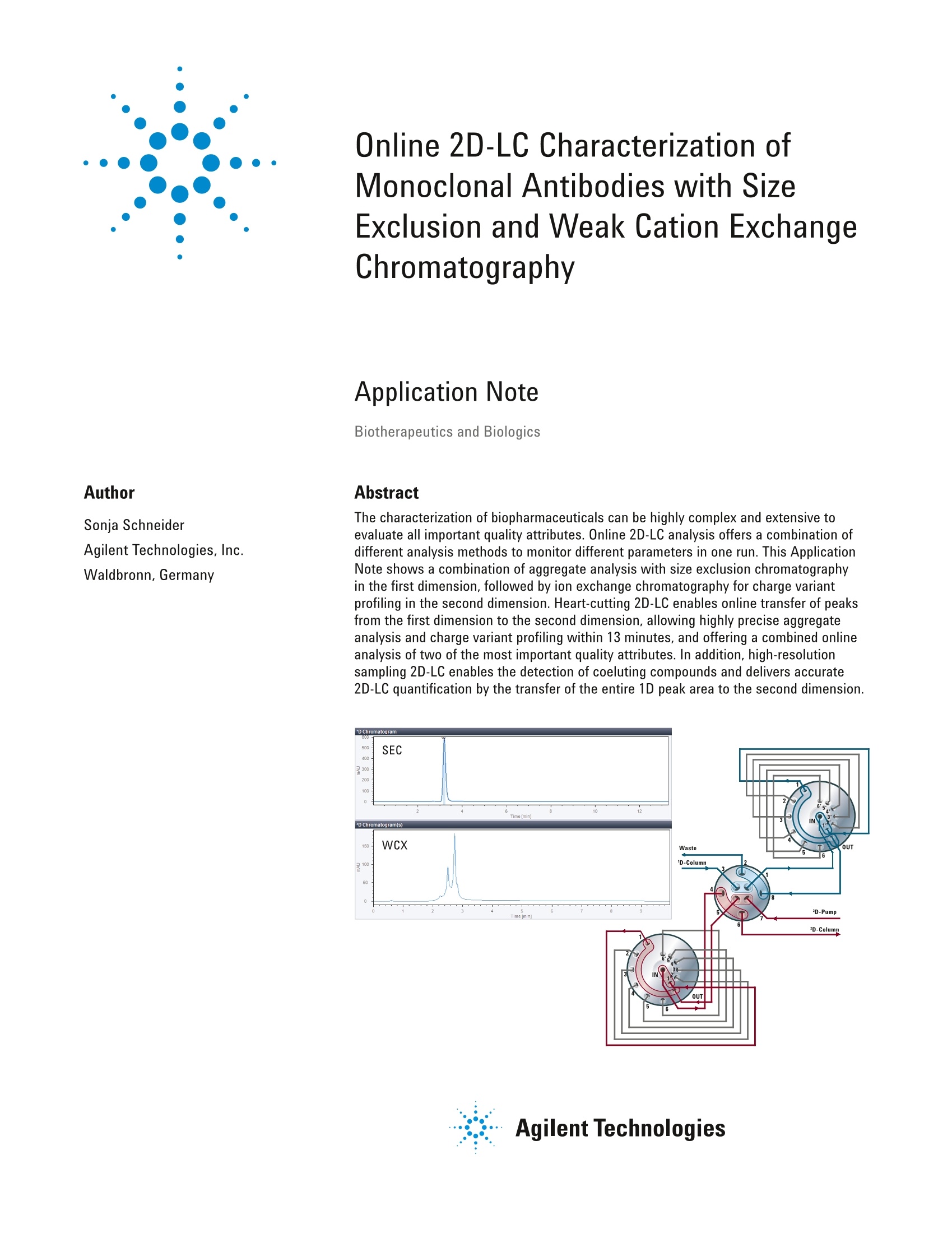
Online 2D-LC Characterization ofMonoclonal Antibodies with SizeExclusion and Weak Cation ExchangeChromatography Application Note Biotherapeutics and Biologics Author Abstract Sonja SchneiderAgilent Technologies, Inc. Waldbronn, Germany The characterization of biopharmaceuticals can be highly complex and extensive toevaluate all important quality attributes. Online 2D-LC analysis offers a combination ofdifferent analysis methods to monitor different parameters in one run. This ApplicationNote shows a combination of aggregate analysis with size exclusion chromatographyin the first dimension, followed by ion exchange chromatography for charge variantprofiling in the second dimension. Heart-cutting 2D-LC enables online transfer of peaksfrom the first dimension to the second dimension, allowing highly precise aggregateanalysis and charge variant profiling within 13 minutes, and offering a combined onlineanalysis of two of the most important quality attributes. In addition, high-resolutionsampling 2D-LC enables the detection of coeluting compounds and delivers accurate2D-LC quantification by the transfer of the entire 1D peak area to the second dimension. Agilent Technologies Introduction Detailed and in-depth characterizationof biopharmaceuticals is of extremeimportance to ensure their safety andefficacy from triggering unpredictableimmunogenic responses. Differentparameters have to be monitored duringvarious stages in the development andproduction of the product. In early phasesof process development, an enormousnumber of samples need to be screened.Those extensive studies include importantquality attributes such as titer analysis,aggregation studies, charge variant andglycan profiling, peptide mapping, andmany others. Due to the extent andcomplexity of the used methods, fullcharacterization of biopharmaceuticalsis an elaborative, time-consuming, andcost-intense business. Two of the mostimportant quality attributes in monoclonalantibodies (mAbs) are aggregation andcharge variant analysis . With the Agilent 1290 Infinity II2D-LCsolution, two analysis types can becombined in an online setup to increaseefficiency and reduce hands-on time(no fraction collection and reinjectionnecessary). In addition to the twoclassical 2D-LC modes: (multiple)heart-cutting and comprehensive 2D-LC,acting complementary to each other,high-resolution sampling 2D-LC combinesadvantages of both heart-cuttingand comprehensive 2D-LC. Up to 10consecutive cuts can be defined for thefirst dimension with the same valve setupthat is used for multiple heart-cutting2D-LC2. Especially in biochromatographysuch as affinity chromatography, sizeexclusion (SEC), or ion exchangechromatography (IEX), high-resolutionsampling can be helpful by enablingthe analysis of larger areas of interestor broad unresolved peaks in the firstdimension. This Application Note shows thecombination of aggregation analysisusing SEC with weak cation exchangechromatography (WCX) for a subsequentcharge variant profiling of an mAb usingheart-cutting 2D-LC for a short onlineanalysis in 13 minutes. In addition,high-resolution sampling offers the optionto transfer the complete 1D peak to thesecond dimension to identify potentialcoeluting impurities as well as to enableaccurate 2D-LC quantification. Experimental Instrumentation The Agilent 1290 Infinity lI 2D-LC Solutionwas comprised of the following modules: First Dimension Pump:Agilent 1260 Infinity Bio-InertQuaternary Pump (G5611A) Second Dimension Pump:Agilent 1290 Infinity Il High-SpeedPump (G7120A) Agilent 1290 Infinity IlMultisampler (G7167B) with cooler Agilent 1290 Infinity lI MulticolumnThermostat (G7116B) Agilent 1290 Infinity Valve Drive(G1170A) with 2-position/4-portduo-valve (2D-LC) valve head(G4236A) 2x Agilent 1290 Infinity ValveDrives (G1170A) with 2x multipleheart-cutting valves (G4242-64000)equipped with 40-pL loops 2x Agilent 1290 Infinity II DiodeArray Detectora (G7117B) with10-mm Max-Light cartridge cell(G4212-60008) Agilent AdvanceBio SEC300A, 4.6×150 mm, 2.7 pm(p/n PL1580-3301) Agilent Bio MAb, non-porous,4.6×50 mm, 1.7 pm,stainless steel (p/n5190-2401) Software Agilent OpenLab CDS ChemStationEdition software, version C.01.07 [27]with Agilent 1290 Infinity 2D-LC software,version A.01.03 Solvents and samples All solvents used were LC grade.Fresh ultrapure water was obtainedfrom a Milli-QIntegral system equippedwith a 0.22-pm membrane point-of-usecartridge (Millipak). Sodium chloride andmonobasic and dibasic sodium phosphatewere purchased from Sigma-Aldrich,St. Louis,MO,USA. An mAb solution was injected in a 50 mMphosphate buffer, pH 6.2(10 mg/mL).It was filtered using an Agilent CaptivaPremium Syringe Filter, regeneratedcellulose membrane, 15-mm diameter,0.45 pm pore size (p/n 5190-5109). Results and Discussion An mAb was analyzed for aggregatesusing the Agilent AdvanceBio SEC 300Ain the first dimension. Almost baselineseparated, a small aggregate peakwas detected in front of the main peak(Figure 1 inset). After integration, anaggregate amount of 1 % was calculated. Parameter Value First-dimension parameters 1D column Agilent AdvanceBio SEC 300 A 1D mobile phase 50 mM Phosphate buffer, pH 6.2 1D flow rate 0.5 mL/min Second-dimension parameters Mode Heart cutting and High-resolution sampling 2D column Agilent Bio mAb 2D mobile phase A) 25 mM Phosphate buffer, pH 6.2 B)500 mM Sodium chloride in 25 mM phosphate buffer, pH 6.2 2D gradient stop time 6 minutes 2D cycle time 10 minutes 2D flow rate 0.5 mL/min 2D gradient 0.00 minutes-5% B 5 minutes-30 % B 6 minutes-40%B 2D time segments Heart cutting Time 1D 3.13 Mode Time based Sampling time 0.11 High-resolution sampling Time 1D 3.05 Mode Time based Sampling timee5 seconds Cuts 8 Injection volume 5 pL Thermostat autosampler 6°C Column temperature RT DADs 280 nm, 4 nm, Ref. 360 nm, 100 nm Peak widths 0.025 minutes (0.5 seconds response time) (10 Hz) Figure 1. Aggregate analysis after size exclusion chromatography using an Agilent AdvanceBio SEC300A, 4.6×150 mm, 2.7 um column. A heart-cut from the main peak wastransferred to the second dimensionfor charge variant analysis using asub-2 pm column, the Agilent BioMAb, nonporous, 4.6×50 mm, 1.7 pmfor optimal resolution in a short runtime. Figure 2 shows the 2D-LC Viewerwith the first dimension (aggregateanalysis) in the upper chromatogram,where the heart-cut that is transferredinto the second dimension for charge variant analysis (lower chromatogram)is marked. In addition to the main peak,the charge variant analysis reveals twoacidic and one basic variant. Acidic andbasic species are defined based on theirretention times (RTs) relative to the mainpeak. The variants eluting before the mainpeak in cation exchange chromatographyare defined as acidic, whereas thevariants eluting later than the main peakare defined as basic3. The short (50-mm) sub-2 um Agilent BioMAb column enables highly resolvingcharge variant analysis in the seconddimension, even for shorter run times.Compared to traditional charge variantanalyses with 30 to 40 minutes run timeson 250-mm columns (5-um particle size),here, a total run time of 10 minutes isenabled. The cycle time for the complete2D-LC run was approximately 13 minutes,including column regeneration. Figure 2. 2D-LC viewer with SEC aggregate analysis in the first dimension (A), and WCX charge variant analysis in the second dimension (2D signal, B). The 2Dpeak table (C) allows a closer look into the second dimension chromatogram. The marked compound in the 2D peak table is shown as the blue region in the 2Dchromatogram. The green and the red arrows mark the integrated area in the second dimension. Precision of retention time and areawas determined in the first and seconddimension for seven consecutiveinjections. In the first dimension, theaggregate and main peak were evaluated.Four peaks were evaluated in the seconddimension: two acidic variants, one mainpeak, and one basic variant. Figure 3shows an overlay of seven consecutive Figure 3. Overlay of seven consecutive 2D-LC runs: 1D aggregate analysis and 2D charge variant analysis. To check for coeluting impurities afterSEC analysis, the main peak was sampledinto eight cuts using high-resolutionsampling to include the complete peakfrom the first dimension (Figure 4). Nocoeluting impurities were detectedafter WCX in the second dimension,as all resulting eight chromatograms(eight cuts) were similar, only differing insignal intensity. Figure 4. The 2D-LC analysis with SEC aggregate analysis in the first dimension, showing the chosen HiRes sampling set of cuts for subsequentcharge variant analysis in the second dimension. No coeluting impurities were detected. Aggregation and charge variant analysiswere combined using the Agilent 1290Infinity ⅡI 2D-LC solution for the analysisof mAbs. High resolution and excellentprecision was found for both dimensionsin a short combined SEC-WCX runwithin 13 minutes total cycle time usingheart-cutting 2D-LC chromatography. Thenumber of aggregates was determined tobe approximately 1 % of the main peak.Charge variant analysis in the seconddimension revealed two acidic and onebasic variant, in addition to the mainpeak. Potential coeluting impurities couldideally be detected using high-resolutionsampling where the relatively broadpeak from the first SEC dimension wassampled in eight cuts, and transferred tothe second dimension. In this case, nocoeluting peaks were detected after WCXanalysis in the second dimension. References 1. ICH, ICH Topic Q6B Specifications:Test Procedures and AcceptanceCriteria for Biotechnological/Biological Products ( 2. Access A gilent Newsletter March 2016: http://www.agilent. com/en-us/newsletters/ accessagilent/2016/mar/2D- LChrs?cid=12822 ) ( 3. DDu, Y.; et al. Chromatographic analysisof the acidic and basic species of recombinant monoclonal antibodies, MAbs Sep 1 2012, 4(5),578-585. ) 4. SSchneider, S. Simple MethodOptimization in mAb Charge VariantAnalysis using pH GradientsGenerated from Buffer Advisorwith Online pH and ConductivityMonitoring, Agilent TechnologiesApplication Note, publication number5991-3365EN, 2014. www.agilent.com/chem For Research Use Only. Not for use in diagnosticprocedures. This information is subject to change without notice. C Agilent Technologies, Inc., 2016Published in the USA, June 1, 20165991-6906EN Agilent Technologies 生物药物的表征可能非常复杂且广泛,以评估所有重要的质量属性。 在线2D-LC分析提供了多种不同的分析方法的组合,可在一次运行中监控不同的参数。 本应用指南显示了聚集分析与一维尺寸排阻色谱的结合,然后是离子交换色谱第二维电荷变化分析的组合。 中心切割2D-LC可将峰从第一维在线转移到第二维,从而允许在13分钟内进行高精度的聚集分析和电荷变化谱分析,并提供两种最重要的质量属性的组合在线分析。 此外,高分辨率采样2D-LC可检测共洗脱化合物并提供准确的通过将整个1D峰面积转移到第二维进行2D-LC定量。
关闭-
1/8
-
2/8

还剩6页未读,是否继续阅读?
继续免费阅读全文产品配置单
安捷伦科技(中国)有限公司为您提供《生物药物中单克隆抗体检测方案(液相色谱仪)》,该方案主要用于治疗类生物药品中含量测定检测,参考标准《暂无》,《生物药物中单克隆抗体检测方案(液相色谱仪)》用到的仪器有安捷伦 1290 Infinity 二元液相色谱系统(1290 LC)。
我要纠错
推荐专场
相关方案






 咨询
咨询