方案详情文
智能文字提取功能测试中
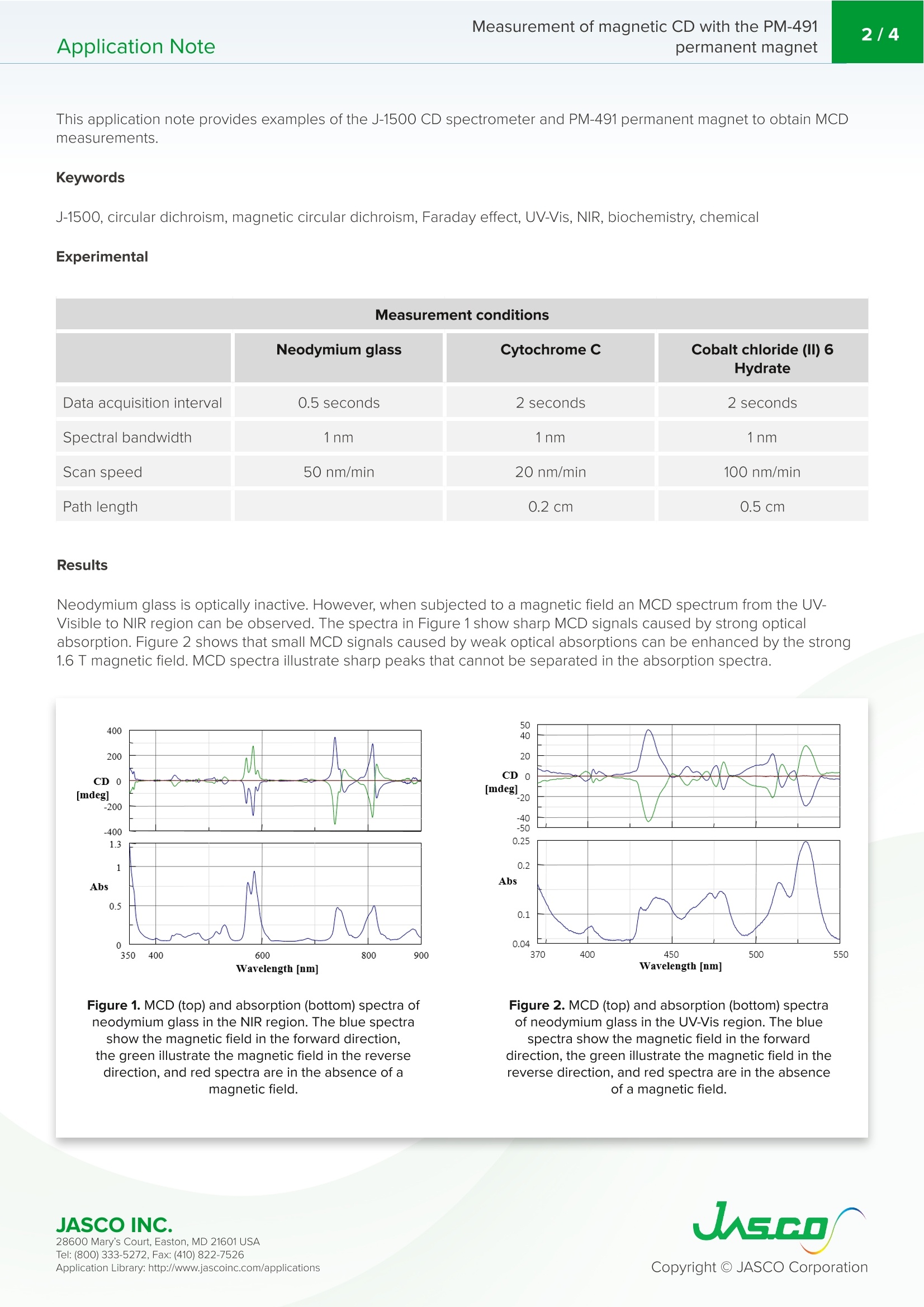
ORD和CD可以用来观察这些磁场诱导的光学活性分子。这些技术通常被称为磁光学旋转色散(MORD)和磁圆二色性(MCD)。如今,MCD被广泛用于探测蛋白质分子的局部环境。由旋转对称性(芳烃、卟啉)、不成对自旋(金属络合物)或两者(血红素)产生的具有大磁矩的发色团对电子扰动敏感,因此提供了有关分子电子状态的信息。MCD信号强度与磁场强度成比例,磁场强度可以通过使用永磁体来施加。以前,为了产生强磁场(大于1特斯拉),需要一个大电磁铁。然而,这种类型的电磁体由于其过大的重量(通常大于60kg)而不能容易地设置在CD光谱仪的样品舱内。最近,JASCO推出了PM-491永磁体。PM-491的磁场为1.6特斯拉,结构紧凑,因此很容易安装在分光偏振仪样品舱内。磁场的方向可以通过简单地反转磁体的方向来改变。本应用说明,J-1500 CD光谱仪和PM-491永磁体可用于获得各种样品的磁圆二色光谱。Application Note Measurement of magnetic CD with the PM-491permanent magnet2/4Application Note CDS Spect r oscopy Measurement of magnetic CDwith the PM-491permanent magne t Whe n l in early po l a ri ze d light is passed th r ough a material in a m ag n et i c fie l d parall e l to the di rec t io n o f the m ag n eti c f ield, th e p o lariza ti o n pl a n e i s r ot a t ed . The u s e o f a ma g ne tic f i eld t o i nd u ce optical activ i ty in mole c u l es i s also known as the F a ra da y e f fe c t . Faraday effect ORD and C D c an b e u se d to obse r ve these magnet i c f i e ld-i n du c ed opti ca lly a c t iv e mol ec u l es . Th es e te c h n i q ue s a re more c om mo n ly k n own a s m a gn eti c o p ti ca l ro t a ry d i s pers i o n (MORD) a n d m ag n etic circ u la r d i chrois m (MCD).MCD i s w id e ly u s e d t od ay t o pr o b e the l o c a l e n v ir o nmen t of pro t ei n mol ec u les . C h rom o pho res wi th l a r ge mag ne t i c m ome n ts ar is i n g from e i ther rotational symmetr i es (aro m a ti cs , p orp h y r in s ), u n p aire d s pi n s (me t a l c om p lex e s),or bo th (heme s) ar e s ens i tiv e t o elec tro ni c pe r tu rb at i o n s a n d therefore p rov i de i nfo r m atio n regar d ing th e m o l ec u le's e l ectr o n i c s t a te. T h e MCD s ign a l in t e n sity i s propo rt i o n a l t o the ma g n e t ic f ie l d stre ngt h, w hi c h c an b e a pplied by u s i ng a p e r ma n ent ma g ne t . Pr ev i o u sly, in or d er to genera t e a strong m a gne t i c fie l d (g r ea t e r t h a n 1 Tes l a ) a lar g e e l ectro m a gn et w as req uir ed .H o we v er , th i s typ e o f el ectromagn et ca n n o t easi l y b e se t in side the samp l e compa r tme n t of a CD spec tr ometer due t o i t s ex cess iv e we i gh t (typ i c all y gr ea t e r tha n 60 k g ).R e c en tly J ASCO h as in trod u c ed th e PM-491 p e r ma n e n t m ag n et . T h e PM-491 ha s a magnet i c f ie l d o f 1.6 Tesla a n d i s c o mp a c t s o it easi ly f i ts i nsid e the s pe ctro po larimeter sa mple compartmen t. The d ir ecti o n o f t he m a gn eti c f i el d ca n b e changed by s i mply revers in g the direc ti o n of the magnet. 28600 M ary's C our t, Easton, M D 21601 US A This application n ote p rovide s example s o f th e J-1500 CD s p ect r ometer an d P M -491 p e r ma n ent magnet to o b tai n MCD m e as u r e m en t s . Keywords J-1500, c i rc u la r d i chro i s m ,ma g n etic c irc u la r d i chro i s m, F a r ada y effe c t, U V -Vi s , NIR, bi o c h e m i s t r y , che m ical Experimental Measurement conditions Neodymium glass Cytochrome C Cobalt chloride (II) 6Hydrate Data acquisition interval 0.5 seconds 2 seconds 2 seconds Spectral bandwidth 1nm 1nm 1nm Scan speed 50 nm/min 20 nm/min 100 nm/min Path length 0.2 cm 0.5 cm Results Neodymium g lass i s optic a ll y in active. Ho w ever , w h e n s ubj ected to a m ag n etic f ield a n MCD spec tru m from the U V-Vi s ibl e to NIR region ca n b e obse r ve d . T h e sp ec t r a in F i g ure 1 sh o w sh a r p MC D si g n a l s ca u sed b y st r on g o p ti ca l abso r pti o n. F i g ur e 2 s h o w s th at sm a ll MCD sign a ls ca u sed by weak o p tical ab s o rp t i ons can be en h a n ce d by the stron g 1.6 T ma g ne t i c f i eld. MCD s p ectra i llu s tr ate shar p p eaks th at ca n n ot b e se p a r ated in the abso r p tion s p ectr a . Figure 1. MCD (top) and absorption (bottom) spectra of neodymium glass i n the NIR region. The blue spectra show the magnetic f ield in the forward direction,the green il l ustrate the magnetic field in the reverse direction, and red spectra are in the absence of a magnetic field. Figure 2. MCD (top) and absorption (bottom) spectra of neodymium glass in the UV-Vis region. The blue spectra show the magnetic field in the forward direction, the green il l ustrate the magnetic field in the reverse direction, and red spectra are in the absence of a magnetic field. I f th e d ir ecti o n o f th e m a g n etic fie l d is the same as th at of the incoming l i g h t, the magne ti c f i eld ca n be de f ined as be in g in th e f o r w a r d d i r ec t io n . If th e d i r e c ti o n o f t h e m ag n e t i c f ield i s o pp o s it e t o th at of the i n c o min g l i g h t, i t i s d e f i n e d a s th e magn eti c f i e ld i n th e re v e r s e d ir e c t i o n . Th is i s d e pi cte d i n Fi g u r e 3. B y c han gi n g th e d i r e c t io n of th e magn eti c f ie ld ,t he MCD s p ectra can also b e reversed. Figure 3. The magnetic field in the forward direction (lef t ) and the magnetic field in the reverse direction (r i ght). MCD of Fe (I II) cytochrome c MCD is wide l y use d for the str u ctu r a l an al y si s o f hemopro t e i n sa m p l es and severa l studies of myoglobin', hemoglobin ,cy t ochro m e b s, c y toch r ome c 3.5-6, c yt ochrome P-450', an d horse r a d i s h p erox i d as e ° are repor t ed . T h e M CD spectra o f the S o r e t b a n d o f F e(I Il ) c yto ch r om e i n s o lut i on a r e sh own in Fi g u r e 4. I f t he CD s p ectru m ob t a i ned i n th e ab sen c e of a magne ti c f i eld is subt r acte d from the M C D s p ectra, sym metr ic MCD spec tr a can be observed . Figure 4. MCD spectra of Fe(IIl) cytochrome c before (lef t ) and after (right) subtraction of the CD spectrum. The blue spectra IIL show t he magnetic fie l d in the forward direction, the green illustrate the magnetic field in the reverse direction, and red spectra are in the absence of a magnetic f ield. MCD of cobalt chloride (Il) 6 hydrate A n aque o u s solu ti o n o f c o b a lt ch l or i de (II) 6 hy d ra t e e xi sts a s a h e x ac oo r d i na t ed s t ructu r e w h e re th e co b a lt i o n i s coor d inated wi th six wa t er m o l ec u l e s . Co n ve r s e l y, co b a l t chlor ide 6 hyd r ate in a co n cen tr ate d HCI sol u tion exists as a t etrahedron comp l ex i n w h i c h the co b a lt i on i s coordin a ted t o fo u r ch l or i de ions. T h e MCD spect r a ill u s tra t e d r a s tic c ha ng es based on th e chan g e in th e e l ectron i c sta t e o f th e co b a lt i o n and a re sho w n in F i g ure s 5 and 6 28600 Mary's Cour t , E asto n , MD 21601US A Figure 5. MCD spectra of cobalt chloride (II) 6 hydrate in aqueous solution. The bl u e spectra show the magnetic field in the forward direction, the green illustrate the magnetic f ield in the reverse direction,and red spectra are in the absence of a magnetic field. Figure 6. MCD spectra of cobalt chloride (II) 6 hydrate in concentrated hydrochloric acid sol u tion. The blue spectra show the magnetic field i n the forward direction, the green illustrate the magnetic field in the reverse direction, and red spectra are in the absence of a magnetic field. Conclusion This application n ote i llu s tr ates th a t the J -1500 CD spectrometer a n d the PM-491 p erm an e n t magnet ca n b e u sed to o bt a in ma g ne ti c c irc u la r d i chro i s m sp ectr a fo r a v ar i e t y o f sa mple s . References 1. V i c k e ry , L ., Noza w a , T ., a nd K. Sa u e r, J ACS (1976), 98,343-350. 2. Y os h i d a, S., Liz u k a , T ., Noza wa , T . a nd M. Hatan o , Bio c h i m . Bioph y s. Act a .(1975), 405,122-135 3. Vickery , L., Noz a w a , T ., and K. S a uer, JACS (1976), 98, 351-359. 4. Vicker y , L ., Salmon, A. G ., a n d K. Saue r, B i ochim. Bio ph ys.A c t a .(1975),386,87-98. 5. Bri a t, B., Ber g er , D., and M . Leli b oux, J . Chem . Ph y s . (1972), 57,5606. 6. Ko b aya s hi, N., N o z a wa, T., a nd M. Hat a no , Bull . Ch e m. Soc . Jpn . (1981), 54,919-924. 7. Sh imi zu , T., Nozaw a , T ., Hatano, M., l m ai, Y.,a n d R . Sato, Bio c hem i str y (1975), 14,4173. 8. Noz a wa, T ., Ko b a y as h i , N., a n d M . H a t ano, B i oc h i m . B i ophys. Ac t a . (1976),427,652-662. 9. Schooley , D. A ., Bun n enber g , E., an d C. Dje ra s s i , C h emist r y (1965), 53,579-586. JASCO INC. 28600 Mary's Cour t , E asto n , MD 21601US A
关闭-
1/4

-
2/4
还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
佳士科商贸有限公司为您提供《使用PMCD-586永磁体的磁性圆二色性》,该方案主要用于其他中磁圆二色光谱检测,参考标准《暂无》,《使用PMCD-586永磁体的磁性圆二色性》用到的仪器有JASCO圆二色光谱仪CD J-1500。
我要纠错
相关方案






 咨询
咨询





