

我在故我思
第1楼2006/11/21
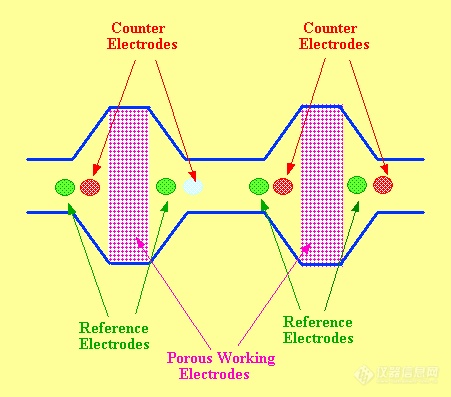
Each electrode unit has a central porous carbon electrode, on either side of which is situated a reference electrode and a auxiliary electrode. As the pressure drop across the porous electrode is relatively small, these electrode units can be connected in series forming an array. Up to 16 units can be placed in series and these arrays are commercially available. However, a sensor system that contains as many as 80 electrodes in the space of a few millimeters has also been constructed (53). A progressively greater potential is applied sequentially to the electrodes of each consecutive unit. This results in all the solutes migrating through the array until each reaches the unit that has the required potential to permit its oxidation or reduction. 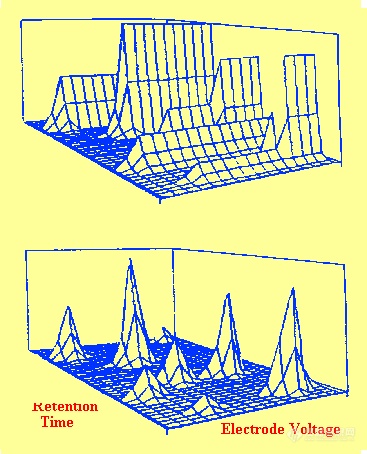
Figure 61 Three Dimensional Graphs Demonstrating the Difference between Amperometric and Coulometric Detection Employing an Electrode Array
A comparison between amperometric and coulometric detection is shown in figure 61. In amperometric detection only a small part of the solute is reacted and so the remainder can proceed to the next electrode system and be detected again. In this way each of the units will detect all the solutes and the graph shown in the upper part of the figure is produced. It is seen that there is no discrimination by the different electrode voltages. Coulometric detection results in the total solute being reacted and thus it will be detected by that unit that has the required potential and not be sensed by other units. It follows that distinct peaks will be produced only at those units with the appropriate potentials. The result is a three dimensional graph similar to that shown in the lower part of the figure. It is seen that each unit produces a peak at a potential that is characteristic of the solute being detected.
我在故我思
第2楼2006/11/21
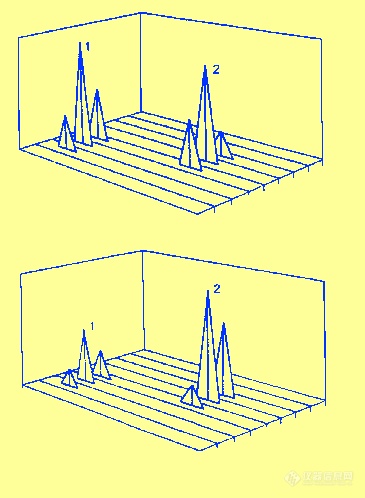
Figure 62. Use of the Electrochemical Array to Confirm Compound Identity
Although the voltage increases progressively from one unit to the next, reaction is not completed at one unit only. This is because reaction will not take place at a specific potential, but over a narrow range of potentials. In practice the signal is usually detected over three contiguous electrode units. For example, the first will oxidize a very small amount of the solute, the second unit will be the dominant unit and oxidize the majority of the solute, while the third unit will oxidize the small remaining quantity of solute. This produces a characteristic pattern of peaks for a particular solute. The ratio of peak height for the three contiguous electrode units that sense the substance will be different for different substances although they may be reacted at the same three electrodes. This is obviously a method of confirming the identity of the solute and is demonstrated in figure 62. The upper graph shows the reference chromatogram of two standards each showing specific retention times and a specific peak pattern as provided by the electrode array detector. The lower graph is for a similar sample and, although the retention times for the pair of solutes is very similar, the pattern given by the electrode array detector clearly shows that the second compound eluted is not the same as that of the second standard.
我在故我思
第3楼2006/11/21
The electrode array detector also gives improved apparent chromatographic resolution in a similar way to that of the diode array detector. Two peaks that have not been chromatographically resolved and are eluted together can still be shown as two peaks that are resolved electrochemically and can be quantitatively estimated. Another advantage is that high oxidation potentials can be used without the high background currents and noise that usually accompany such operating conditions. The electrodes that are operating at high voltages are "buffered" by the previous electrodes operating at lower voltages which results in reduced background currents and noise.
Another example of the application of the detector to the separation of a number of neuroactive substances (54)is shown in figure 63. It is seen that for certain applications the electrochemical array detector can be extremely useful.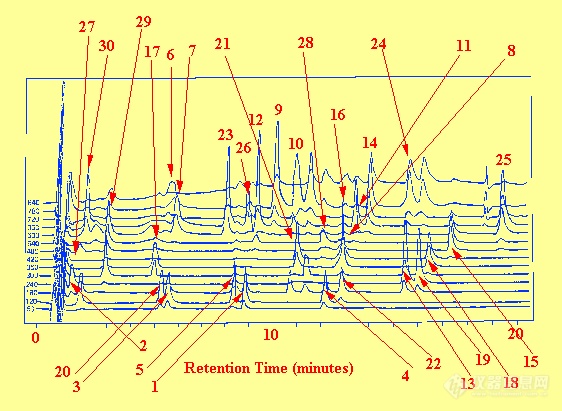
Mobile Phase 1% Methanol to 40% Methanol in a Phosphate (0.1 mol l-1 buffer with ion pairing (pH 3.4)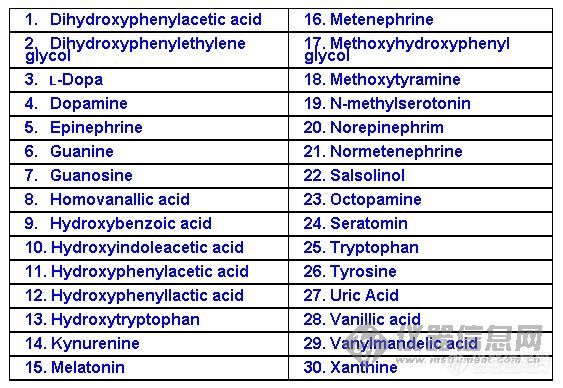
Figure 11 The Separation of 30 Neuroactive Substances Monitored by an Electrochemical Array
Nevertheless, in order to use the detector, the solutes must be amenable to electrochemical reaction and capable of being separated using a mobile phase that will conduct an ion current