


在自动积分后,对称的峰有可接受的基线;
增强峰、或削减峰的目的是为了满足系统适用性接受标准,或满足放行质量标准。
有水有渝
第2楼2018/11/12
色谱积分原理和手动积分的规则
原创: GMP办公室翻译组 GMP办公室 2016-10-02
Questions of Quality: Where Can I Draw The Line?质量问题:我能在哪里划这条线?A question that keeps raising its head when working in a regulated laboratory is can chromatographers integrate peaks manually? If they can, when can they do it? Also if they can manually integrate, when should they not do it?一个问题正在持续发酵并且占据了头条:在受控实验室何时能手动去对色谱峰积分?如果他们能这样做,何时可以做呢?同样如果他们能够手动积分,何时不能这样做呢?

One of the red rags to a regulatory bull is the issue of manual integration of chromatograms. If seen during an inspection, you can almost see the wheels in the inspector’s brain turn in mechanical precision as they question: are they testing into compliance? In a 2014 FDA inspection of a laboratory in Europe, one FDA 483 observation stated:色谱手动积分问题就像是斗牛中的一块红布。如果在检查期间被发现,你几乎能够看到检查官的脑中条件反射地联想到一个问题:他们的检验是否合规?在2014年FDA检查一个欧洲试验室时,一个FDA483的缺陷项如下: No procedure exists describing how to perform manual integration.没有关于怎样进行手动积分的规程。 A more serious non-compliance occurred in the FDA warning letter to Leiner Health Products:在对Leiner Health Products 的FDA警告信中提到一个更加严重的不符合项: In addition, our investigators documented many instances with extensive manipulation of data with no explanation regarding why the manipulation was conducted. This manipulation would include changing integration parameters or relabelling peaks such that previously resolved peaks would not be integrated and included in the calculation for impurities.
此外,我们调查人员记录了许多关于修改数据的案例,他们都没有解释为什么修改。这些修改包括改变积分参数或者重新标定峰以至于原来峰的分离度不能进行积分和杂质计算。
There have been many other cases that I summarized in a recent Questions of Quality column on the role of chromatography data systems (CDS) in data falsification. What regulatory guidance is there to help us? We need to consider some basics of integrating chromatographic peaks before we can look at manual integration and the regulatory guidance surrounding it. The reason for this is simple: if integration parameters are set correctly and the chromatography is acceptable then there should be no need to reintegrate manually in many cases. However, we also need to acknowledge that chromatographic systems are dynamic by their nature and separations can change during a run, so getting the right overall integration can be a balancing act.还有一些其他情况,我近期总结了关于一个色谱数据系统(CDS)在数据造假中的作用的质量问题专栏。在这里有什么法规指南能够帮助我们?我们在关注相关积分手册和法规指南之前需要先了解一些色谱积分的基础问题。这样做的理由很简单:如果正确设定积分参数和可接受的色谱图,那么一些情况就没有必要进行手动重新积分。但是,我们需要承认在检测运行期间色谱系统的特性是动态的和分离是可变的,因此正确全面的设定积分能起到平衡的作用。 The regulators are wrong in their requirement for a procedure on manual integration. What is required instead is a standard operating procedure (SOP) for chromatographic integration, of which manual integration is an important sub-set. Although the focus of this column is manual integration please do not lose sight of the bigger picture. As such, we will not be discussing the various calibration methods that could be applied to standards to quantify analytes in samples nor will we be looking at analogue to digital conversion because the latter has been covered in an earlier Questions of Quality column . Furthermore we will not be considering the use of system suitability tests (SSTs), again because this column has already discussed them . The paper by Hill et al. on manual reintegration in bioanalysis is also a highly recommended publication to read on the subject.监管者对手动积分规程的要求是错误的。这些要求对色谱积分的标准操作规程(SOP),而不是手动积分的要求,手动积分是另外一个重要分支。虽然专栏的焦点是手动积分,但是我们要比较全面的来看待问题。因此我们将不讨论适用于标定样品定量分析使用的各种校验方法,也不去关注类似的数据转化因为这些已经在之前的质量问题专栏中提到过了。此外我们也将不考虑系统适用性(SSTs)的使用,因为已经再其他期的专栏中讨论过了.本文强烈推荐去阅读Hill et al关于生物分析的手动重新积分的课题. Back to Integration Basics
色谱积分基础
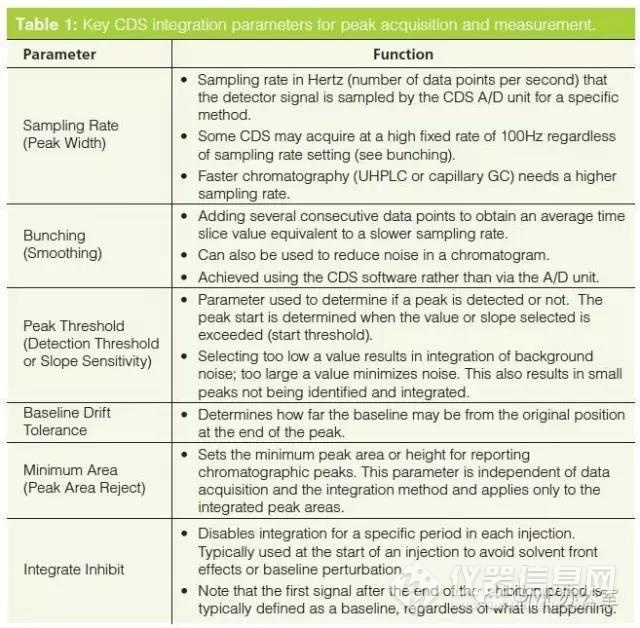
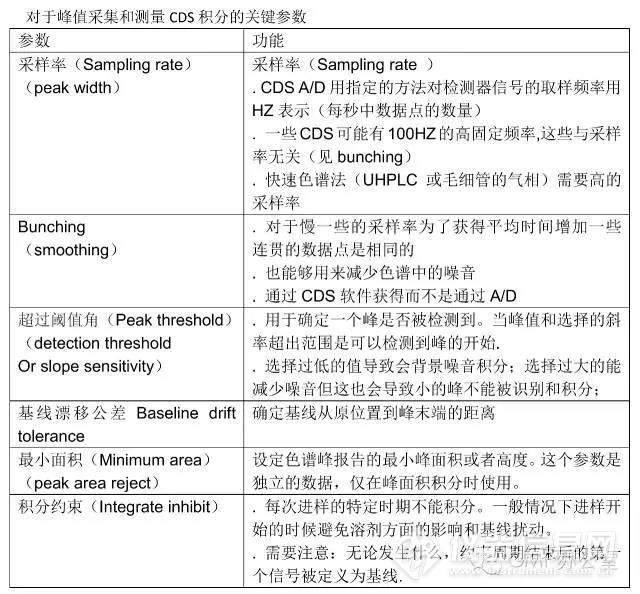
Before we can discuss how to control and manage manual integration it is important to understand the basics of chromatographic integration itself. The best book on the subject of integration is by Norman Dyson and, although the second edition was published in 1998, it is still applicable and highly recommended if you want to understand chromatographic integration. The key integration parameters are shown in Table 1 and the main ones are discussed below. Note that some CDS suppliers may call these parameters a different name but the functionality is essentially the same.之前我们讨论如何控制和管理手动积分,这对于色谱积分本身基础的理解是十分重要的。对于积分这个课题最好的书是Norman Dyson 的著作,在1998年已经发行了第二版,它仍旧使用。如果你想了解色谱积分强烈推荐这本书。关键积分参数在表1中列出,并且一些重要的部分在下面进行了讨论。值得注意的是不同的CDS供应商对他们的参数有不同名称,但是功能的本质是相同的。
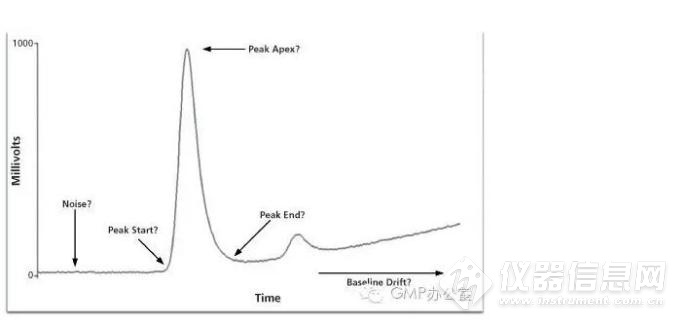
图1:峰的关键参数
对于峰值采集和测量CDS积分的关键参数
Chromatographic integration begins when the CDS samples the detector output using an analogue to digital converter (A/D). An integration method in the CDS determines the frequency of the detector data collection and analysis run time. Dependent on the CDS, either an integration method or a processing method will be automatically applied to the file of data slices to calculate the peak areas or heights. The processing method will contain the identities of the peaks of interest and their expected elution time windows. These data files and their interpretation constitute part of the raw data and primary record for the analysis. We will discuss the definition of primary record in the next Questions of Quality column in light of the two MHRA data integrity guidance documents issued this year .
当检测器用一个类似于数字转换器(A/D)的装置开始输出信号时色谱积分开始。在CDS中积分的方法确定了检测器收集频率和分析运行时间。依靠CDS,积分方法或处理方法将自动应用于峰面积或高度的计算积分。处理方法包括对目标峰的鉴别和期望在界面中的洗脱时间。这些数据文件和解释组成了分析的原始数据和最初记录。我们将讨论在下一个质量问题专栏中讨论最初记录的定义,根据MHRA发布的数据完整性指南。
The two most important parameters for integration are sampling rate and peak threshold. In combination they determine the slices for peak measurement but also suppress noise to allow better peak measurement .
采样率和超过阈值角是积分的两个最重要参数。结合他们两个决定了峰测量,但抑制噪声能够使峰得到更好的测量。
Peak width or sampling rate setting governs how often the detector output is sampled, which directly impacts the accuracy of peak area measurement. If the sampling rate is too slow it could result in the integration missing a small fast eluting peak or a valley between two peaks. Conversely, too fast a data acquisition rate can be managed by data bunching in the CDS software. Typically you should sample a peak at least 20-30 times across its width so that it can be integrated accurately; for example, conventional high performance liquid chromatography (HPLC) typically requires a 0.5-1 Hz sampling rate, faster capillary gas chromatography (GC) has a sampling rate in the range 5-20 Hz, and ultrahigh-pressure liquid chromatography (UHPLC) needs a sampling rate between 20-50 Hz. Most A/D units in CDS are rated at up to a 100 Hz sampling rate.
切线峰宽(peak width)和采样率的设定控制检测器采样的输出频率,并对峰面积测量的准确度有直接影响。如果采样率太慢,可能导致积分不到小的洗脱峰和两峰之间的低谷。相反,太快的采样频率会被CDS软件中的数据聚束所管制。一般情况下,至少20-30次采样才能准确的积分。例如,传统HPLC一般要求0.5-1HZ的采样率,快速毛细管气相GC的采样率范围5-20HZ,UHPLC的采样率范围是20-50HZ.大多数在CDS中的A/D中的采样率上限是100HZ.

有水有渝
第3楼2018/11/12
手动积分规则——手动积分还是手动干预?
GMP办公室翻译组
作者:Bob McDowall, "Questions of Quality" column editor for LCgc Europe
翻译:Lily
校对:Owen
What is Manual Integration?
什么是手动积分?
So far we have discussed the regulatory issues around peak integration, the main integration parameters, and the three rules of integration so that you have a fighting chance for automatically integrating your peaks and there is no need to intercede manually. However, we now need to turn our attention to manual integration. As we can see from the regulatory citations and the bioanalytical guidance documents, what is needed is an SOP to control, manage, and authorize integration — both automatic and manual.我们已经讨论了关于峰积分发布的相关法规,主要的积分参数,和重新积分的三个原则,满足这些我们能对峰进行自动积分而没必要进行手动积分。但是,现在我们需要把注意力转向手动积分。正如我们从法规引文和生物分析指南文件看到的,我们需要的是控制、管理批准自动/手动积分的SOP。 In an ideal world there would be a definition of manual integration. However, there is not a definition available for manual integration that I could find. Neither in Dyson, nor on any regulatory website. This was the point made by Hill et al. (7), who discussed the scope and terminology of manual reintegration and they concluded that it is essential to develop a consensus with respect to definitions. The problem, they noted, is that that a consensus does not exist.手动积分的定义应该确定。但是我没有找到一个可以使用的关于手动积分的定义。无论是Dyson还是在所有法规网站中都没有。Hill et al. (7) 中提到过,他讨论了手动积分的范围和专业术语,他们都说有必要对其定义进行统一。问题是,没有统一。 This raises an important question: if we can’t define manual integration how can we write an SOP on integration as a whole?这就出现了一个重要问题:如果我们不能定义手动积分,怎样在整体上编写一个关于积分的SOP? Scope of an Integration SOP 积分SOP的范围?
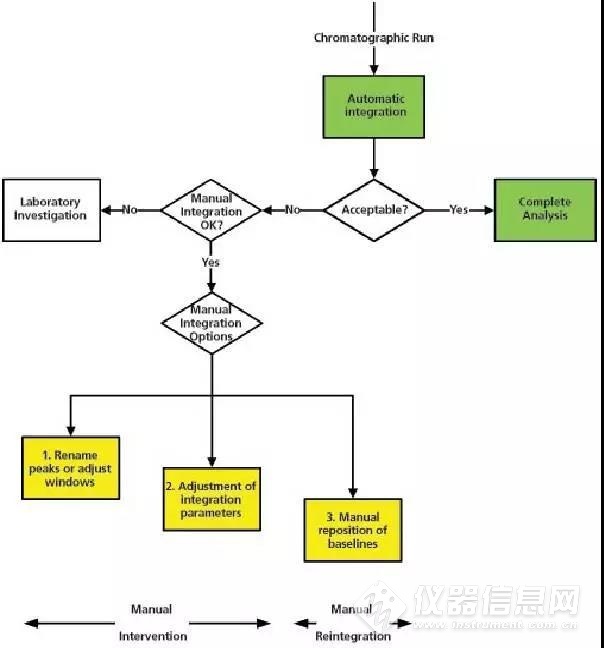
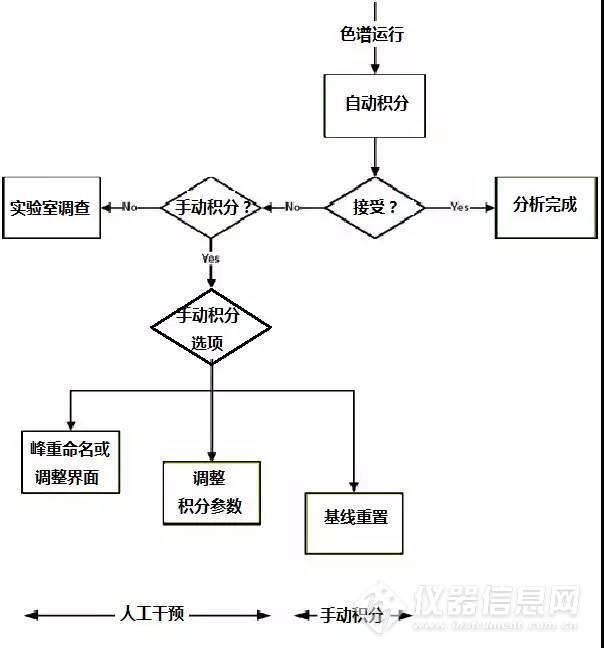
In the spirit of the early pioneers, this column will present a possible approach to writing an SOP in the form of a flowchart that will consider some, but not all, options for both automatic and manual integration. Of course, there is a little trepidation because one definition of pioneer is finding new and exotic ways to die (!) However, I hope that this column and my views stimulate a debate about what constitutes manual integration and we can get a definition agreed upon.本着拓荒者的精神,这个专栏将提出一个可能的方法来编写SOP,以流程图的形式讨论了手动积分和自动积分的一些选项。当然,这有一些担忧,因为拓荒就是作死的节奏啊。但是,我希望这个专栏和我的观点能够引发一个关于手动积分的大讨论,使我们能够得到一个统一的定义。 In this discussion we need to balance sound science with regulatory compliance. Therefore, let me pose a question — which is worse: not reintegrating when you know the results are wrong or accepting wrong results when you can see an unknown peak in the sample chromatograms? You see the dilemma? We know the outcomes of both situations are undesirable, but as a result of inflexible procedures or fear of the regulations sound scientific judgement cannot be exercised.在这个讨论中我们需要平衡各种科学合理的法规符合性。因此,让我们提出一个问题看看一下哪一个更糟糕:当你知道结果错误的时候没有重新积分,或者你看到样品色谱图中有未知峰,然而你接受了错误的结果?你看到困境了吗?我们知道这些情况是不可取的,但是死板的程序或者对法规的担忧使得科学判断不能行驶。 First, let us consider a “no manual integration allowed” option. Personally, I think that this is an untenable situation, particularly if a regulated laboratory is using recently developed methods, analyzing impurities where the limits of quantification or detection are close to baseline noise, or investigating stability of products. In these situations, manual reintegration is scientifically sound and defendable provided that it is covered in the integration SOP.首先,让我思考一个“不允许手动积分”的观点,个人认为这是一个站不住脚的观点,特别是受控的实验室使用近期开发的方法分析杂质,且定量限或检测限接近基线噪音,或者在研究产品的稳定性时。这些情况手动积分都是科学合理的并且在积分SOP中可以涵盖的。 A suggested flowchart for consideration of integration is shown in Figure 3. It begins with the completion of the chromatographic run and the automatic integration of peaks by the original processing method. The resulting chromatograms are reviewed by the chromatographer to see if retention times and peak shapes are as expected, the peak(s) have been correctly identified, baseline placement is as required by the analytical procedure, and the sample is integrated consistently with the standard. There may be other criteria that an individual laboratory wishes to apply. We now come to a decision point: is the run acceptable? If yes the individual results and the reportable value are calculated and all is well. All data at this point have been calculated automatically by the CDS, the chromatographer is merely confirming what the software has done conforms to pre-defined expectations.在图3是关于积分考虑的建议性流程图。它在色谱运行完成时开始,通过初始处理方法进行峰的自动积分。色谱工作人员对色谱结果进行复核:保留时间和峰的性状都是否和预期一致?峰是否能够被正确鉴别?基线位置是否符合分析程序?样品按照标准进行积分?个别实验室可能有其他标准。现在我们需要决策:运行是否可接受?如果是,就计算各个的结果和报告的数值,一切都好。所有数据都通过CDS进行自动计算,色谱工作人员仅需要确认软件所做的符合预期期望就可以了。 However, if the integration is not acceptable we move to a second decision point: is manual integration (whatever that term may cover) permitted for this analytical procedure? If not the next stage is a laboratory investigation. What methods could we consider for inclusion for no manual integration? Perhaps measurement of active pharmaceutical ingredients (APIs) or registered methods for finished product for the active ingredient? If these peaks cannot be integrated correctly are you out of control? We will consider if this is tenable when we have finished discussing the flowchart.但是,如果积分不能接受我们需要进行第二个决策:该分析程序是否允许手动积分(无论该术语包含哪些内容)?如果不能,下一阶段就是实验室调查。我们应该考虑使用哪些方法不能手动积分吗?如果是APIs(active pharmaceutical ingredients)的检测或对最终产品的活性成分的注册方法呢?如果这些峰不能被正确积分,你就没辙吗?我们将在完成这个流程图讨论的时候思考这是否站得住脚? In my view, manual integration must be specifically prohibited in the following circumstances:我认为,在以下情况手动积分是禁止使用的。
有水有渝
第4楼2018/11/12
高效液相色谱图积分问题探讨(转载)
药群论坛 2017-09-27
高效液相色谱图怎样积分?积分规则是什么?
高效液相色谱图都是用图像处理软件来实现积分。
积分规则为:同一次测定的对照品和样品的色谱图最好是在设定一个固定的积分条件进行积分计算,积分参数一致的情况下进行可减少人为主观意识上的误差。
效液相色谱图又称“高压液相色谱”、“高速液相色谱”、“高分离度液相色谱”、“近代柱色谱”等。高效液相色谱是色谱法的一个重要分支,以液体为流动相,采用高压输液系统,将具有不同极性的单一溶剂或不同比例的混合溶剂、缓冲液等流动相泵入装有固定相的色谱柱,在柱内各成分被分离后,进入检测器进行检测,从而实现对试样的分析。
FDA对HPLC手动积分怎么看?
实验室数据完整性已成FDA检查制药企业的一把利器,其中一条关于随意手动积分或者无书面程序规定手动积分,说明了FDA本身并不是不认可手动积分,而是要求有操作规程规定什么情况可以手动积分,如何积分,并且应保存并记录积分参数。
CFDA紧跟FDA的步伐,在2016年度多次飞检中,开具多条数据完整性方面的缺陷,收回多张GMP证书。关于手动积分并没有开具缺陷,制药企业应防患于未然,提前把手动积分管理好。
一、关于手动积分的指南
关于手动积分,并没有指南给出明确的定义。笔者只查到有两篇生物样本分析的方法验证指南规定了再积分(Reintegration)的注意事项。
FDA的2001年定稿指南 Bioanalytical Method Validation,及相应的2013年草案指南也有类似的要求。 生物样本分析的方法验证2013草案指南,第III.C节“样本数据再积分”规定:SOP应该确定再积分的标准,和怎样进行再积分;应清楚描述、并记录再积分的理由;应报告原始和再积分数据。第VII.D节还规定:再积分数据应有管理人员授权再积分。
EMA的2011年指南 Guideline on bioanalytical method validation,第5.5节规定:应该有SOP描述色谱的积分和再积分。应在分析报告中讨论偏离这个SOP的任何偏差。应该记录色谱积分、再积分的积分参数、初始积分数据、最终积分数据,并在要求时立即可得。
二、什么情况下可以进行手动积分?
1)低分离度或低响应;
2)流动相不稳定,基线紊乱,保留时间变化小;
3)运行过程中,出现异常峰;
4)软件积分的局限性,如由于软件参数阈值的设置导致峰未积分,峰起点和终点间出现肩峰和异常的基线漂移,软件错误(峰未积分和错误识别峰);
5)由于样品母体的干扰导致的复杂色谱图:裂峰、目标杂质的同时洗脱/肩峰、基线噪音、负峰、基线上升或下降(由于某些梯度程序)、峰的严重拖尾、烃类的存在导致自动积分不正确。
三、手动积分的权限及操作
操作员在数据系统自动积分不正确时,领取手动积分处理报告表并填写,手动积分申请被QC经理批准后由QC经理或QC组长(若有)或其指定人员(一般是指经验丰富的资深QC)方可进行手动积分。
四、手动积分注意事项
1)手动积分不能试图通过以下动作来使数据符合标准要求:减少峰(减少峰面积);增加峰(增加峰面积);改变峰高;
2)同一次检验的所有标准品、工作对照品、样品等必须使用同一方法进行手动积分。
3)手动积分结束后,应打印自动积分图谱和手动积分图谱附于手动积分申请表之后,提交QC经理审核;QC经理应复核完整的数据找出根因,基于根因制定相应的CAPA措施来避免类似事件。
4)自动积分的图谱不得删除或被覆盖,应与手动积分图谱保存于计算机中;打印的自动积分图谱和手动积分图谱应标识清楚签名,随同手动积分申请表附于检验原始记录后面一起交质量管理部经理、QP审核无问题后归档。
五、色谱软件选型、权限设置和结果显示
满足合规要求的色谱软件(例如Waters和Angilent的软件),能够分别设置不同权限,例如普通化验员不具备进行手动积分的权限,需要主管进行操作,有些软件则不具备这些功能;即使是同一个品牌的软件,也有不同版本;即使软件有这个功能,企业也不一定启用。
有两个信息是否显示,对色谱数据透明度非常重要:方法名称和数据版本。如果企业不使用手动积分(重画基线),而采用修改参数的方式,则每修改一次,是不同的方法(规范的管理程序会要求更改方法名称保存,否则就只能到审计追踪才能查出来这个方法已经被修改了),而打印的色谱图可以选择是否显示方法; 每处理一次数据,会形成一个数据版本,同样的,打印的色谱图可以选择是否显示数据版本。一个完全透明的结果显示,会在打印的色谱图上显示“方法名称+数据版本”(同时要求修改参数则改变方法名称,不需要QA或检查员仔细检查审计追踪才能发现修改痕迹)。