






第1楼2005/03/25
Table 8.1 contains intensity data collected from Figure 8.5. This table shows:
(A) the concentration of Cd;
(B) the relative concentration of As to Cd;
(C) the net intensity of the corresponding Cd concentration with no As present;
(D) the estimated standard deviation of measurement of Cd;
(E) the net intensity of 100 ppm As at the 228.802 nm wavelength;
(F) the estimated standard deviation for measurement of As;
(G) the estimated standard deviation of the combined signals for As at 100 ppm and Cd at the concentrations given;
(H) the uncorrected relative error for measuring Cd 228.802 nm with 100 ppm As present, and;
(I) the best-case relative errors for correcting the Cd intensity to account for 100 ppm As.
Table 8.1:
Estimated Errors of As on Cd 228.802 nm line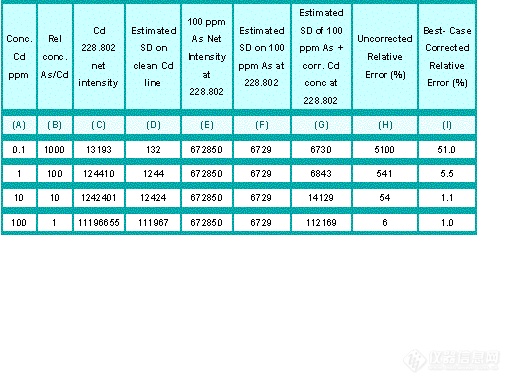
It was assumed that the precision (expressed above as standard deviation) of measuring the intensity of the As or Cd contributions at 228.802 nm is 1%. In addition, it was assumed that the best-case precision for making a correction is calculated using the following equation:
SDcorrection = [(SDCd I)2 + (SDAs I)2]1/2
where:
SDcorrection = standard deviation of the corrected Cd intensity;
SDCd I= standard deviation of the Cd intensity at 228.802 nm;
SDAs I = standard deviation of the As intensity at 228.802 nm
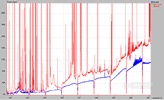
We can see from the above assumptions that a very optimistic view was taken in making this assessment. If we assume that a best-case detection limit for Cd at 228.802 nm in the presence of 100 ppm As would be 2 x SDcorrection, then the calculated detection limit is 0.1 ppm. In reality, the detection limit would be closer to .5 ppm. The detection limit for the Cd 228.802 nm line is 0.004 ppm (spectrally clean) showing roughly a 100-fold loss. Furthermore, the lower limit of quantitation has been increased form 0.04 ppm (10 x the DL) to somewhere between 1 and more realistically 5 ppm Cd. Figure 8.6 illustrates the situation with the spectra of 1 and 10ppm Cd solutions with and without 100 ppm As present.
Figure 8.6:
1 and 10 ppm Cd with and without 100 ppm As
Correcting for the interference of As upon Cd would require that (1) the As concentration in the solution be measured and that (2) the analyst already have measured the counts/ppm As at the 228.802 nm line (sometimes called correction coefficient). This information allows for a correction by subtracting the calculated intensity contribution of As upon the 228.802 nm Cd line, thereby making the correction. This approach further assumes that slight changes in the instrumental operating parameters and conditions will influence both the analyte (Cd) and the interfering element (As) equally (i.e., an assumption many analysts are not willing to make).
The problems associated with direct spectral overlap make it difficult for the analyst to perform quantitative measurements. Each case should be reviewed. If a spectral correction is found to be necessary, the reader is advised to consult their operating manual where a defined procedure will be outlined using the instrument抯 software.
Types: ICP-MS
The types of spectral interferences most commonly encountered for ICP-MS are discussed in the Spectral Interferences section of our Reliable Measurements series. You may wish to review this information before continuing.
Avoidance: ICP-MS
The following are possible avoidance pathways:
The use of high resolution ICP-MS.
Matrix alteration through elimination ?for example, elimination of S, and halogen containing reagents such as Cl.
The use of reaction and or collision cells to destroy molecular interfering ions.
Cool plasma to reduce background interferences.
Separation of analyte(s) ?for example the use of chromatography or solvent extraction, etc.
The use of alternate ICP discharges such as He, mixed gas (He-Ar, N2-Ar, etc.).
Low-Pressure ICP discharges.
The above approaches are just examples of some of the approaches that have been taken to avoid interferences. For a given application, it is suggested that a literature search be performed in an attempt to benefit form the vast amount of research that has been conducted in this area. In addition, instrument manufacturers are constantly revising and updating their instrumentation and software in an attempt to take advantage of new technologies. Thus, consulting with the manufacturer may help when interferences are encountered.
The fact is that the mass spectra of elements are much less detailed than in optical emission spectroscopy. Most elements have fewer than seven isotopes and many have only one (monoisotopic). When interference is encountered, it may be possible to go to another isotope even if it is less abundant. The difficulty in obtaining low detection limits in ICP-MS with interference correction is a function of the relative signal intensities and measurement precision as illustrated above for ICP-OES. If a correction cannot be avoided, many analysts seek alternate techniques rather than run the risk of reporting unreliable data.