


第1楼2005/03/30
Tips for the Analyst:
Keep the volume of reagents to a minimum.
Keep sample preparation and measurement times to a minimum (use timers and don't let a digestate sit overnight or even during a lunch break).
Keep a history of beakers and other apparatus as to chemical exposure.
Exercise care in handling reagents (exposure to atmosphere or pouring liquid back into the same container or into the wrong container can result in serious contamination).
Disposable powder-free gloves prevent contamination from earlier exposures as opposed to reusable gloves (vinyl is better for difficult manipulations such as pipeting and handling wet glassware while latex is more rugged).
Disposable laboratory coats prevent contamination from earlier exposures.
Disposable foot coverings keep any dirt that the 憇ticky mat' missed contained.
NOTE: Dangerous operations require appropriate laboratory clothing REGARDLESS of the potential for contamination.
Apparatus Contamination
Another potential source of contamination is casued by the apparatus that comes into contact with the sample. Careful planning is required by the analyst as to the type of apparatus that comes into contact with a particular trace metals analysis. All apparatus should be specified in the procedure along with any cleaning procedures or special precautions. Although the type and degree of contamination that occurs when using certain apparatus can be predicted, the importance of one or more blanks with every preparation should be apparent from the following table.
Table 10.1: Results of Spectrochemical Analysis
(Hydrogen Fluoride, Hydrogen Chloride, and Nitric Acid [all high purity] analyzed after evaportation in Teflon, Platinum, and Quartz dishes)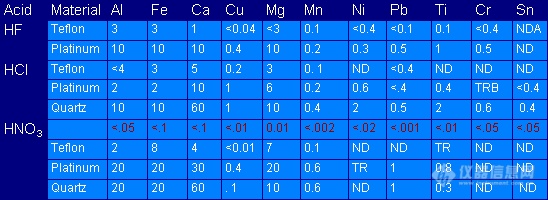
Elements determined, ng/g
ND = Not detected
TR = Trace, not evaluated quantitatively
第2楼2005/03/30
A Closer Look at Quartz:
Quartz is a popular material for apparatuses used in trace elemental analysis. There are two types of quartz: 1) opaque and 2) transparent.
Opaque quartz has the highest trace element concentration and should not be used for trace analysis.
Transparent quartz (types I and II) are made from naturally occurring quartz crystals or sands. Type I is made by electric melting and type II by flame melting. Type II has slightly less impurities than type I (some impurities are volatilized by the flame). Type III quartz is made synthetically by vapor phase hydrolysis of pure silicon compounds such as SiCl4. This type of quartz is more pure than the natural quartz with the exception of Cl - which is ~ 50 ppm. Type IV quartz is synthetically made from SiCl4 using a process involving electrical fusion of the oxidized staring material. It is as pure as type III, with respect to trace metal content, and contains much more Cl-. Use synthetic quartz whenever possible.
Table 10.2 shows typical impurities in natural and synthetic quartz.
Table 10.2: Recorded Impurity Levels in Types of Quartz1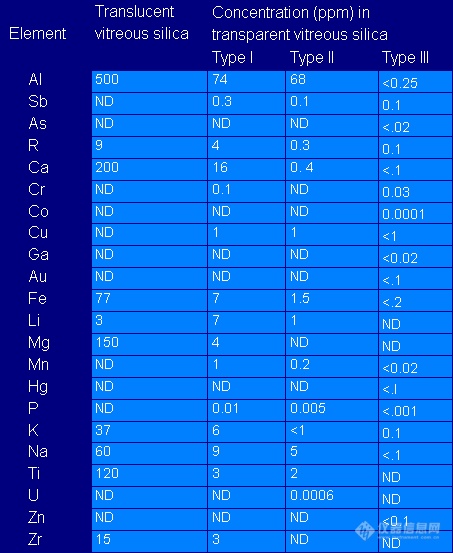
ND = Not detected